Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Four-Square Nitrogen
Chemists provide definitive evidence for the first tetrazetidine, a four-membered nitrogen ring
by Stephen K. Ritter
July 16, 2012
| A version of this story appeared in
Volume 90, Issue 29
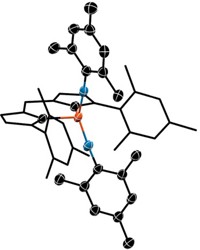
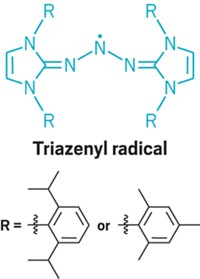
Australian chemists have spotted what they say is the first definitive example of a tetrazetidine, a molecule containing an unprecedented four-membered nitrogen ring (J. Am. Chem. Soc., DOI: 10.1021/ja303019y). The tetrazetidine system is one of the few simple chemical structures that has not yet been synthesized with any certainty. Over the past 20 years, David Camp and Ian D. Jenkins of Griffith University and Graeme R. Hanson of the University of Queensland have used electron paramagnetic resonance spectroscopy to study the formation of radicals when triphenylphosphine and diisopropyl azodicarboxylate are combined in the Mitsunobu reaction. In addition to a phosphine-azodicarboxylate radical, they sometimes noticed a second persistent radical with what they describe as a “rather beautiful, almost symmetrical nine-line spectrum.” Now, in conjunction with Griffith’s Marc Campitelli, and using more advanced and affordable computational methods, the researchers believe the tetrazetidinetetracarboxylate radical cation shown is being formed by a Michael-type addition of a phosphine-azodicarboxylate radical to a diisopropyl azodicarboxylate molecule. The tetrazetidine radical is surprisingly long-lived, they note, lasting several hours at room temperature.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter