Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Taking A Different Tack With Amyloid-beta
Research team designs brain-penetrating compounds that may indirectly reduce production of the Alzheimer’s peptide
by Lauren K. Wolf
August 13, 2012
| A version of this story appeared in
Volume 90, Issue 33
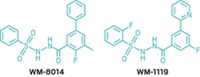
Inhibiting the formation of amyloid-β, the peptide that clumps in the brains of patients with Alzheimer’s disease, has been a focus of drug designers for years. Rather than regulating amyloid-β directly, researchers at the University of Pennsylvania, led by Kurt R. Brunden, are now trying to control its production indirectly via an oxidative stress pathway in the body (ACS Chem. Neurosci., DOI: 10.1021/cn3000795). Neuroscientists have known that when amyloid-β clumps deposit in the brain, the peptide aggregates trigger formation of oxidized lipids such as isoprostane-2αIII. The Penn team,which also included chemists Carlo Ballatore and Amos B. Smith III, designed small molecules to block the thromboxane A2-prostanoid (TP) receptor, a membrane protein whose activation by isoprostane-2αIII leads to an increase in amyloid-β production. Compounds that inhibit the TP receptor already existed, but they had a hard time penetrating the blood-brain barrier. By replacing a carboxylic acid on one of those molecules with a nonacidic oxazole or thiazole group, the researchers synthesized a batch of lead compounds (one shown) that entered the brain in mice and bound tightly to both human and mouse TP receptors in cells.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter