Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Nickel Appends Aqueous Fluoride For Radiotracer Synthesis
Nickel catalysts mediate oxidative fluorination reaction that could help make imaging agents
by Bethany Halford
October 22, 2012
| A version of this story appeared in
Volume 90, Issue 43
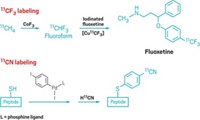
The 110-minute half-life of 18F doesn’t give researchers much time when they want to incorporate the isotope into radiotracers for positron emission tomography. A new oxidative fluorination reaction allows chemists to add aqueous fluoride to arenes and alkenes in one step, thereby circumventing the time-consuming fluoride drying procedures typically used in radiotracer synthesis (J. Am. Chem. Soc., DOI: 10.1021/ja3084797). The reaction, developed by Eunsung Lee and Tobias Ritter of Harvard University, along with Jacob M. Hooker of Massachusetts General Hospital, uses arylnickel catalysts in combination with a hypervalent iodine oxidant and aqueous fluoride (example shown). “The transformation represents the first example of fluorination with a first-row transition-metal organometallic and the first example of a reaction that affords aryl fluoride by 18F–C bond formation at room temperature within seconds using an aqueous solution of 18F fluoride,” the researchers note. They point out that because the synthesis is relatively simple, it should be easy for radiotracer makers to adopt.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter