Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Greened-Up Copper Addition Reactions
Micellar system enables sensitive organometallic reagents to carry out C–C couplings in water
by Stephen K. Ritter
December 17, 2012
| A version of this story appeared in
Volume 90, Issue 51
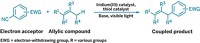
There was once a time when chemists took extra precautions to keep air and moisture out of organometallic reactions, thinking that the reagents were overly sensitive to proton quenching and/or oxidation. But those days are gone. In one of the latest examples, a research team led by Bruce H. Lipshutz of the University of California, Santa Barbara, has created a reaction system to forge new C–C bonds using organocopper chemistry in water at room temperature (J. Am. Chem. Soc., DOI: 10.1021/ja309409e). The researchers take an alkyl halide and an α,β-unsaturated ketone and mix them together with zinc powder, a copper salt, and gold trichloride in water containing TPGS-750-M, a commercial “green” surfactant designed by Lipshutz’ group as part of its effort to avoid using organic solvents. Within the micelles that form, an in situ-generated organozinc intermediate transfers its alkyl group to copper. Copper then completes the task of adding the alkyl group in a 1,4-fashion to an enone substrate, a process assisted by AuCl3, which serves as a Lewis acid promoter. The micellar system eliminates concerns about the water sensitivity of the organozinc and organocopper reagents, Lipshutz says, plus provides broader reaction scope and higher yields than water-based, radical-induced 1,4-additions studied in the past.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter