Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Cascade Beats Previous Record By Four Steps
Organic Synthesis: Four starting materials plus 12 steps in one pot, et voilà—bioactive agents
by Stu Borman
January 4, 2012
A cascade of reactions consisting of 12 steps has produced complex natural-product-like molecules, some of which have promising biological activities. The achievement could lead to new drug discovery programs based on the class of molecules produced, according to a Nature Chemical Biology paper about the work (DOI: 10.1038/nchembio.758).
In cascade chemistry, a mixture of just the right reagents takes relatively simple starting materials and catalysts through multiple reaction steps to produce more-complex molecules, without the need for laborious protection and deprotection steps or intermediate purifications. Cascades involve at least two separate reaction steps that proceed in a single reaction flask.
Cascade processes with as many as eight steps were developed previously. Now, a group led by synthetic chemist Kamal Kumar and chemical biologist Herbert Waldmann of the Max Planck Institute for Molecular Physiology, in Dortmund, Germany, and Dortmund University of Technology has created a reaction cascade of unprecedented length.
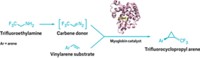
The one-pot, 12-step cascade takes easily accessible triphenylphosphine, alkyne, formyl chromone, and tryptamine starting materials and converts them into complex, natural-product-like indoloquinolizines. Reaction times are a few tens of minutes, and overall yields range from 20% to 91%.
Kumar, Waldmann, and coworkers set out to combine the electrophilic compound benzopyrone with multiple nucleophilic tryptamines to produce indoloquinolizines. However, “the originally expected sequence did not occur,” Waldmann says. “Instead, a longer cascade was set loose. It was quite a puzzle to figure out the overall mechanism,” but he and his coworkers eventually unraveled the cascade’s dozen steps.
Some of the products inhibit cell division by binding to proteins that help form cell organelles called centrosomes and by interfering with centrosome duplication during cell division. The researchers have named the compounds “centrocountins.” They have applied for a patent on the synthesis and the compounds and, along with the Lead Discovery Center, in Dortmund, will further investigate the centrocountins as possible leads for new anticancer agents.
“Accomplishing so many steps in one pot is impressive,” comments synthetic and bioorganic chemist Romano V. A. Orru of VU University, in Amsterdam, whose group developed the earlier eight-step cascade. The new approach also stands out “for the high degree of complexity it generates,” he adds. “People from the drug industry are very interested in this type of chemistry because its high efficiency shortens the synthetic route to highly functionalized molecules dramatically. Cascade reactions are already commonly used in pharmaceutical research to produce heterocyclic fragments for medicinally important molecules,” and the new process expands that repertoire, Orru notes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter