Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
A Flexible Power Source From Soot
Battery Materials: Carbon nanoparticles could form cheap supercapacitor electrodes
by Prachi Patel
January 5, 2012

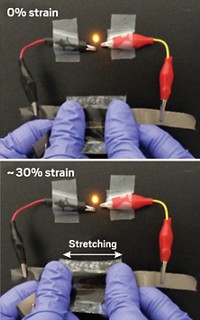
Electronic devices such as displays, sensors, and medical implants are well on their way to becoming flexible. But flexible power sources to operate them are only starting to catch up. Researchers now report a simple method to fabricate bendable supercapacitors that uses carbon nanoparticles from soot (ACS Nano, DOI: 10.1021/nn2041279).
Supercapacitors, like batteries, store energy. While batteries store and release charge through chemical reactions, supercapacitors store it on the surface of their electrodes. Supercapacitors can charge in minutes instead of hours–a fact reflected in their higher power density–and can recharge millions of times. Unfortunately, they hold less energy per weight than batteries. To improve supercapacitors’ energy density, researchers have replaced activated charcoal electrodes with materials with higher surface area, such as carbon nanotubes and graphene, a one atom-thick sheet of carbon. But making and using these materials typically requires complex, costly methods.
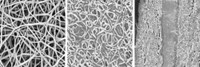
Carbon nanoparticles, on the other hand, are cheap and easy to make, says Zhong Lin Wang, a materials science and engineering professor at Georgia Institute of Technology. He thought that the nanoparticles could form cheap yet effective supercapacitor electrodes. Wang and his colleagues in China started building such electrodes by holding a flexible carbon substrate in front of an ethanol flame for 30 seconds. The flame deposited a thin layer of carbon nanoparticles, each about 7 nm wide, on the fabric. To make the electrode, the researchers then sputtered manganese oxide nanorods on top of the nanoparticle film. Manganese oxide’s high charge storage capacity improves the performance of carbon-based supercapacitor electrodes.
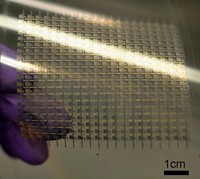
Finally, to make the supercapacitor, the researchers took two of the prepared electrodes, infused them with a polymer gel electrolyte, and sandwiched a standard cellulose separator material between the electrodes.
The resulting foldable device stores 4.8 watt-hours of energy per kg and has a power density of 14 kilowatts per kg. Researchers have achieved higher values with carbon nanotube supercapacitors, but Wang’s supercapacitors should be sufficient to run small devices such as sensors and radio-frequency identification tags, Wang says.
The new electrode shows “solid performance,” says Rodney Ruoff, a mechanical engineering professor at the University of Texas, Austin. “I look forward to seeing more work with these types of materials and electrolytes to see if the energy density can be improved.”
Wang and his colleagues are now stacking multiple supercapacitors to make devices that they hope will store more power. To get higher energy density, they are also tinkering with the size of the carbon nanoparticles, which depends on the distance between the fabric and the ethanol flame.
A handful of startup companies are already trying to commercialize flexible supercapacitors, points out George Grüner, a professor of physics at the University of California, Los Angeles. The success of any of these new technologies will depend on price, he says. “You need these to be disposable and dirt cheap, say 10 cents apiece,” he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter