Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Metal-Free Aromatic Hydrogenations
Organic Synthesis: Lewis acid-base pairs enable unprecedented reduction of anilines and other aromatics
by Stephen K. Ritter
February 23, 2012
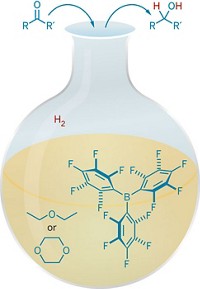
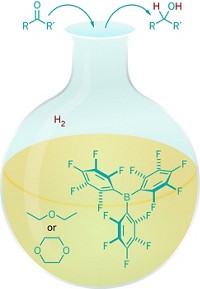
In a chemical first, an international research team has developed a metal-free reaction to hydrogenate aromatic rings to form cyclohexyl derivatives. The achievement could spark a broader range of applications for industrial hydrogenations, which are widely used for processing petroleum and foods.
A metal-free aromatic hydrogenation is surprising, says team leader Douglas W. Stephan of the University of Toronto, because it’s exceedingly hard to overcome the additional stability a molecule gains from aromaticity, even with the best transition-metal catalysts. Stephan and his colleagues have done so by using a chemical construct known as a frustrated Lewis pair, which Stephan introduced in 2006.
Lewis acid-base adducts are common in chemistry: An electron-deficient Lewis acid readily shares a Lewis base’s spare pair of electrons. However, when the Lewis acid and base have bulky substituents, their ability to form a close relationshipr is denied, causing the pair to become “frustrated.”
But the pair still garners penned up reactivity, comparable with that of an organometallic catalyst. Several research groups have shown that frustrated Lewis pairs can intercept and split H2 during an electron tug-of-war and subsequently hydrogenate compounds such as imines, silyl ethers, and N-heterocyclic compounds.
Stephan’s group in collaboration with computational chemist Stefan Grimme at the University of Bonn, in Germany, tackled the hydrogenation of aromatics by using B(C6F5)3 as the Lewis acid and various anilines as the Lewis base (J. Am. Chem. Soc., DOI: 10.1021/ja300228a). When H2 is added, the frustrated Lewis pair splits H2 and then reductively adds hydrogens to aniline’s aromatic ring to form cyclohexylammonium borate salts. Stephan says the salts could be easily deprotonated to release the free cyclohexylamines.
Princeton University’s David W. C. MacMillan, an expert in metal-free organocatalytic reactions, says the reactivity of frustrated Lewis pairs “is conceptually really intriguing” and that the new chemistry “certainly makes one think differently about the notion of aromatic hydrogenations. All in all, this work points to the exceptional fertility of this area for new reactivity discoveries and for mechanistic explorations.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter