Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Putting The Squeeze On Cells
Microfluidics: New device examines cell size and stiffness at once
by Erika Gebel
June 29, 2012

Cells are a bit like pillows: They range in stiffness from firm to soft. Cancer cells are less stiff than normal cells, and stem cells change in rigidity as they differentiate. Unfortunately, current approaches for measuring cell stiffness are either slow or inaccurate. Now researchers have developed a simple microfluidic device that can rapidly and accurately analyze the size and rigidity of a cell (Anal. Chem., DOI: 10.1021/ac300264v).

Scientists can probe a cell’s firmness with atomic force microscopy or optical tweezers, but these methods require skilled operators and lots of time. To analyze large numbers of cells in the clinic or research lab, researchers want a high-throughput means of measurement.
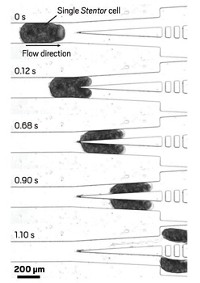
Other research groups have developed fast methods that infer stiffness from the time it takes a cell to travel through a microfluidic channel; a firm cell takes longer to squeeze through tiny passageways than a soft cell does. However, these approaches don’t take cell size into account, says Klavs Jensen of the Massachusetts Institute of Technology. He hypothesized that different-sized cells of the same stiffness should flow at different rates through these tight spaces.
To look at both cell size and rigidity, Jensen, his research associate Andrea Adamo, and the rest of his team built a device consisting of a microfluidic chamber containing two electrodes. A pressurized stream of fluid drives cells through the channel, which in the middle tapers down to a snug path, at a rate of 800 cells per minute, one at a time. Before a cell enters the narrowest section, it moves past a gold electrode. By measuring the electrical resistance between this electrode and one on the other side of the tapered section, the team can determine the cell’s size. Large cells lead to increased resistance. Researchers can measure the cell’s transit time via how long this resistance spike lasts.
The researchers tested their device by sending thousands of HeLa and human fibroblast cells through the channel. Jensen says that electrical resistance measurements reflected cell size measurements the team made using a microscope. Eventually, similar comparisons will enable the researchers to calibrate the device to provide size measurements. To test the device’s ability to analyze stiffness, his team next softened some of the HeLa cells with Latrunculin A, a toxin that breaks down the cytoskeleton. When the scientists compared treated and untreated cells, they found that, as predicted, the soft ones took less travel time on average than the normal ones did. They also found that larger cells were slower on average than smaller ones, fitting Jensen’s prediction.
Dino Di Carlo of the University of California, Los Angeles, applauds the innovation of adding a cell size measurement to cell stiffness assays. Cell size variability “can muck up the data,” he says, from a high-throughput screen of cell stiffness. He points out that friction may also confound these data; cell types may move at different rates because of their interactions with the channel’s surface. Jensen plans to address this issue in future devices.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter