Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Weak Interactions Drive Meta-Substitution Reaction
Catalysis: Strategy opens new doors for C–H activation
by Carmen Drahl
June 27, 2012

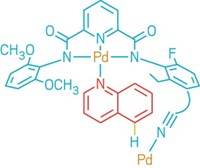
A new mode of C–H activation selectively functionalizes the meta position of aromatic rings, chemists at Scripps Research Institute report (Nature, DOI: 10.1038/nature1158). The strategy could make it easier to bring C–H activation to bear on challenging problems in synthesis, including making drugs with sophisticated substitution patterns.
Despite advances in the activation of inert C–H bonds, it’s still tough to selectively transform one C–H bond among many in an aromatic ring. Chemists’ typical solution is a directing group, which tends to form a rigid six- or seven-membered ring transition state with a metal catalyst and produces ortho-substituted products. Meta substitution has remained elusive because it would require a large ring called a cyclophane in the transition state, which would be strained.
Jin-Quan Yu, postdoctoral researchers Dasheng (Jackson) Leow and Gang Li, and graduate student Tian-Sheng (Tyson) Mei realized they could relieve that strain by designing a less rigid transition state. They devised a nitrile template that coordinates weakly to a palladium catalyst in an “end-on” fashion yet is reactive enough to overcome the entropy in a floppy transition state.
The result is meta functionalization of a variety of toluene derivatives. Adjusting the nitrile template makes it possible to modify hydrocinnamic acid derivatives, which are structural motifs in the multiple myeloma drug Velcade and the blood-pressure-lowering drug Micardis. After the transformation, the team removes the templates through reduction or hydrolysis reactions. The template idea is flexible, Yu says, and it should be adaptable for a wide variety of arenes, including phenols.
Outside of C–H activation, some methods for meta functionalization do exist, explains Victor A. Snieckus of Queens University, who has written a commentary about the work (Nature, DOI: 10.1038/486478a). However, meta substitution on electron-rich aromatic rings remains “a tricky business,” he adds. “Yu’s work offers an intriguing coordination-driven method for selective meta activation,” but the templates will need to be reduced in both size and complexity to make the technique broadly usable, he says.
“This is an interesting and creative strategy,” adds Melanie S. Sanford, whose University of Michigan, Ann Arbor, team develops directed C–H activation methods. In the C–H activation realm, meta functionalization “has proven very, very hard to do in any type of general sense previously, because directing groups are almost universally ortho-selective,” she explains. “The selectivity for the meta position is not yet perfect, but this looks like a very promising start.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter