Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Sunlight May Trigger Nanoparticle Formation
Nanoparticles In The Environment: Organic matter and sunlight reduce metal ions to create nanoparticles, research finds
by Katharine Sanderson
July 31, 2012

Nanoparticles found in the environment could come from natural processes, not just from man-made sources. In rivers and other bodies of water, sunlight can help dissolved organic matter reduce silver and gold ions to form nanoparticles of the metals, according to researchers in China (ACS Nano, DOI: 10.1021/nn302293r).
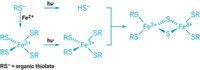
To help understand the fate and environmental impact of engineered particles, scientists want to know more about how nanoparticles, both naturally occurring and anthropogenic, particles behave, says Jingfu Liu at the Chinese Academy of Sciences, Beijing. Liu and his colleagues knew that mercury ions in the environment can get reduced by dissolved organic matter and sunlight, and wondered whether other metal ions would be similarly affected–and in turn might form nanoparticles.
Silver from natural and anthropogenic sources exists as ions in the environment. In their experiment, Liu and his team spiked river water with known concentrations of silver ions. They then exposed the water to sunlight for four hours. Using microscopy and UV-Vis spectroscopy, the researchers found that silver nanoparticles had formed. Similar experiments with gold ions produced gold nanoparticles.
To understand the mechanism, Liu and his team set up a more-controlled system with gold and silver ions, using humic acid as a proxy for nature’s dissolved organic matter. They found that dissolved oxygen plays an important role. When sunlight hits the system, Liu says, the humic acid donates an electron to the dissolved oxygen, which then reduces the ions to their metallic form as nanoparticles.
Liu says the work suggests that it may be difficult to differentiate between natural and engineered nanoparticles. It also shows the extent to which silver and gold can change chemically in the environment, and this should be considered in any future regulation of the uses of nanotechnology, says Liu.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter