Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Fluorescent Probe Highlights CO In Cells
Cell Imaging: New fluorescent probe may help scientists figure out carbon monoxide’s role in biology
by Erika Gebel
September 20, 2012

Carbon monoxide is famous for its toxicity, but recent research suggests that human cells produce the gas themselves. It may even play a critical role in cell signaling. Now researchers report developing a fluorescent probe that produces light upon reacting with CO (J. Am. Chem. Soc., DOI: 10.1021/ja307017b). They say it could allow scientists to study the gas’s nontoxic role in human biology
Scientists have studied other gases such as nitric oxide in human physiology for decades, but CO caught their attention only within the past five to seven years, according to Christopher Chang of the University of California, Berkeley. “There is growing interest and awareness that the body makes it,” says Chang. People don’t know why the body does so, he adds.
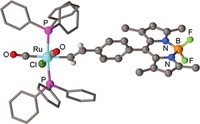
Current methods for detecting cellular CO either destroy the cells or use external devices that, like smoke detectors, catch CO as it wafts out, Chang says. To measure CO inside living cells, Chang and his team needed to develop a probe that binds to it without interacting with other small reactive molecules, such as hydrogen sulfide or nitric oxide. “We looked at classic organic chemistry papers and found it reacted well with metals, specifically palladium,” Chang says. So they designed a palladium-containing probe that releases a fluorescent molecule after it reacts with CO.
To test the probe, the researchers mixed it with seven small reactive molecules common in cells, including nitric oxide. They also mixed it with a molecule that releases CO. The probe lit up only in the presence of the CO-releasing molecule. Next the researchers incubated kidney cells with and without the CO-releasing molecule, and then added the probe to the cells. With a confocal microscope, the researchers observed that cells containing the CO-releasing molecule began to glow within 45 minutes, while the other cells remained dark.
“We are detecting low micromolar to high nanomolar ranges,” says Chang. He does not know whether this detection limit is low enough to detect the CO that cells make naturally, but he hopes that it will be. “The amount that’s produced is unknown,” says Chang.
Designing a probe that selectively reacts with CO isn’t easy, says Mi Hee Lim of the University of Michigan, Ann Arbor, because the gas is not particularly reactive. “They smartly used the metal to capture the carbon monoxide,” she says. “It looks simple but it’s an innovative idea.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter