Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Coaxing Saturated Aldehydes And Ketones To React In An Unexpected Manner
Photoredox catalysis and organocatalysis join forces to connect saturated aldehydes or ketones with aryl cyanides at a spot once deemed inert
by Bethany Halford
April 1, 2013
| A version of this story appeared in
Volume 91, Issue 13
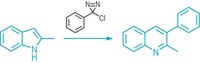
When chemists pick out reactive hot spots on aldehydes and ketones, they generally focus on the electrophilic carbonyl carbon or the α-carbon next door. The α-carbon can easily shed a proton and become nucleophilic thanks to its relationship with the carbonyl. Such neighborliness doesn’t typically extend to the β-carbon just one atom further along, which generally is regarded as being too unreactive in saturated systems. To do anything at the β-position, most chemists aim to install a double bond between it and the α-carbon. David W. C. MacMillan and his team at Princeton University have now found a way to directly arylate the β-position of saturated aldehydes and ketones, opening up an easy path to more elaborate molecules (Science, DOI: 10.1126/science.1232993). The chemists use an iridium-based photoredox catalyst in combination with an organocatalyst containing an amine. The latter forms an enamine with the aldehyde or ketone, which then reacts with the photoredox catalyst to generate a radical. This species reacts with a cyano aryl group, which then eliminates cyanide. Hydrolysis of the amine catalyst finally generates a β-arylated aldehyde or ketone product. MacMillan’s group demonstrated the versatility of the reaction by using a broad range of aldehydes, ketones, and aryl groups (one shown).


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter