Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Fluorine’s Positive Side Revealed
Chemists provide evidence for the first example of a molecule in solution containing a positively charged bridging fluorine atom
by Stephen K. Ritter
April 8, 2013
| A version of this story appeared in
Volume 91, Issue 14
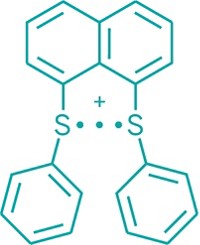
Halonium ions are organic compounds in which a chlorine, bromine, or iodine atom bridges two carbons and is positively charged. They are important synthetic intermediates that have been critical in helping understand how substituent groups on neighboring carbons affect stereochemistry in organic substitution reactions. However, it has not been clear whether fluorine, the lightest of the halogens and the most electronegative element on the periodic table, can form a positive ion in solution in the same manner. But Thomas Lectka and coworkers at Johns Hopkins University have now reported “positive” evidence for the existence of a fluoronium ion (Science, DOI: 10.1126/science.1231247). The trick has been finding a suitable precursor molecule. Lectka’s group came up with a double norbornyl system in which two bridgehead carbons are in proximity to each other. By heating the molecule in aqueous trifluoroethanol, the researchers coaxed fluorine substituted on one carbon to attack the other carbon, forming a bridge. They confirmed the fluoronium ion’s existence via kinetics studies, deuterium-labeling experiments, and computational studies. Lectka says his group hasn’t spent much time yet exploring the reactivity of the short-lived fluoronium ion, but he thinks taming the ion could enable new synthetic methods for making valuable organofluorine compounds.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter