Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Unusual Uranium Species Found In Contaminated Aquifer
ACS Meeting News: Results reveal new chemistry in underground sediments
by Jyllian Kemsley
April 15, 2013
| A version of this story appeared in
Volume 91, Issue 15
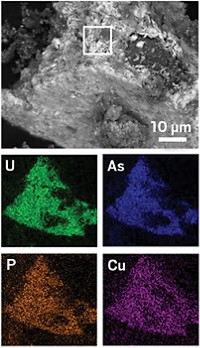
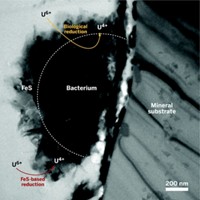
Cleaning up water in aquifers contaminated with uranium from processing ore for nuclear fuels requires understanding the underground chemistry of how uranium is interconverted between water-soluble U6+ and insoluble U4+. Although scientists had believed that all U4+ in aquifer sediments occurred as UO2, new research shows that a combination of microbial and abiotic reactions also produces other U4+ complexes (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.1219198110). One approach to treating uranium-contaminated aquifers is to add a microbial nutrient to create a bacterial bloom that consumes all of the oxygen and creates reducing conditions, said research team leaders John R. Bargar and Noémie Janot of SLAC National Accelerator Laboratory. After using this method on an aquifer in Rifle, Colo., the researchers found that iron sulfide minerals appear to seed on the outside of biomass and reduce U6+ to U4+. The U4+ then may complex with phosphate or other ligands available from nearby biological material. If such ligands are absent, then UO2 may form. The microbes themselves may also reduce U6+ to U4+. The results will change how aquifer behavior is modeled, Bargar said.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter