Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Water
After The Deepwater Horizon Disaster
Components of the giant oil spill went different places with ecological consequences in the Gulf of Mexico
by Jyllian Kemsley
June 3, 2013
| A version of this story appeared in
Volume 91, Issue 22

On April 20, 2010, the Deepwater Horizon oil rig exploded into fire in the northern Gulf of Mexico, approximately 40 miles off the coast of Louisiana. Two days later it sank. The incident killed 11 workers and set off the largest marine oil spill in U.S. history. By the time the well was capped on July 15, 2010, approximately 4.9 million barrels of oil—about 6 × 1011 g—plus another 2 × 1011 g of hydrocarbon gases had gushed out of the undersea Macondo reservoir and into the Gulf.
According to government estimates, a mere 20% of that oil was recovered, either directly from the wellhead or by skimming the sea surface (Proc. Natl. Acad. Sci. USA 2012, DOI: 10.1073/pnas.1214389109). Another 5% was burned, going up in the air, with some turning into particles that drifted to the seafloor. The vast majority of oil—75%—was neither burned nor recovered. It wound up in four places: dissolved in the water, evaporated to the air, stuck to the coastline, or settled on the seafloor.
As for the gas, what was recovered with oil was burned, whereas the rest predominately dissolved in the water column, forming the so-called deep plume.

In many ways, the spill turned out to be far less severe than feared. Federal waters reopened for fishing within a year, and coastal areas largely appear recovered.
Three years after the spill, scientists continue to evaluate what they know—and don’t know—about the largest spill in U.S. history. From a basic science perspective, “it was a fascinating opportunity to see what happens when you perturb a large body of water with a large body of oil,” says Christopher M. Reddy, a scientist at Woods Hole Oceanographic Institution (WHOI). Furthermore, studying how different ecosystems fared in the spill can help identify areas that are particularly tolerant of or resilient to oil, so responders can better direct efforts next time.
Many researchers described their work in this area during the ACS national meeting held in New Orleans in April. The Divisions of Analytical Chemistry, Agricultural & Food Chemistry, Environmental Chemistry, and Geochemistry all organized sessions on oil-spill-related research.
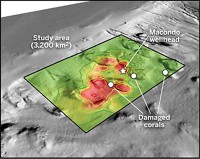
One of the most critical samples collected during the spill was a “ground zero” sample of material from the wellhead that preserved both gas and oil before they mixed with seawater. That sample is the control against which all others are measured. Scientists use it to determine the amount of oil and gas released from the well and to understand how the various hydrocarbon species partitioned and evolved over time, as well as to fingerprint the Macondo oil to identify samples found on the beach or seafloor.
A WHOI team managed to collect two wellhead samples in collaboration with the U.S. Coast Guard. The Coast Guard arranged for the researchers to board a response vessel above the well and use a remotely operated vehicle owned by Houston-based Oceaneering International to collect the material using a gas-tight chamber. After a drama-filled night that included jury-rigging equipment in the glow of flaring gas and one underwater slip of a $40,000 sampler out of a robotic arm—fortunately, the Oceaneering team had thought to tie it to the remotely operated vehicle—the effort succeeded.
Analysis of one of the samples showed that on a molar basis the source Macondo material was 74% saturated hydrocarbons, 16% aromatic hydrocarbons, and 10% polar hydrocarbons (Proc. Natl. Acad. Sci. USA 2012, DOI: 10.1073/pnas.1101242108). The polar compounds incorporate oxygen, nitrogen, and sulfur. Collectively, the compounds spanned a vast range of molecular weights. The single most abundant compound was methane, at more than 80 mole %, followed by ethane, propane, and butane.
The Macondo reservoir lies about 5.5 km below sea level, or 4 km below the seafloor. The approximate pressure was 6,000 psi, and the temperature was 130 °C. As the hydrocarbons moved 4 km up the well pipe to the seafloor, pressure and temperature decreased to 2,200 psi and 4 °C, and the material separated into oil and gas phases. Then, as the oil jetted out of the well, various portions dissolved, aerosolized into droplets, mixed with water to form gas hydrates, or precipitated as waxes.
Overall, these processes meant that some oil compounds, particularly long-chain n-alkanes, likely sank to the seafloor immediately. But the bulk of the material rose up through the water. A deep plume of lighter hydrocarbons, characterized by compounds such as methane, ethane, propane, and benzene, stopped rising about 1 km below the surface because the gas compounds were soluble in water and small oil droplets in the plume were not buoyant enough to rise further.
The remaining thousands of hydrocarbon compounds that make up oil, from C3 to C39 and larger, continued their rise to the surface. There they were skimmed, burned, naturally dispersed through wave action, chemically dispersed, or washed into marshes and onto beaches.
The deep plume attracted a lot of scientific attention during and after the spill because it incorporated so much of the leaking hydrocarbon mass. Although “plume” might conjure images of a river of visible oil 1 km below the surface, in reality it was an area of predominately dissolved compounds in clear water.
Nearly all of the methane released from the Macondo well was trapped in the deep plume, along with much of the ethane and propane. The plume also included significant portions of pentanes and cyclohexane, plus aromatics such as benzene, toluene, and xylenes.
That raised hopes that some of the compounds would be turned into food for creatures that thrive on them. Gulf water contains native hydrocarbon-consuming microorganisms that feed on the oil and gas that naturally seep up from reservoirs under the seafloor. Researchers believed that those microorganisms would use the hydrocarbon influx to eat, grow, and multiply and that the process would rid the water of some or even most of the leaked hydrocarbons. But there was a downside: The booming microbe population would also use up oxygen so that they and higher organisms might die.
Fortunately, the die-off didn’t happen. Various species of microbes did feast on the bounty of hydrocarbons, and dissolved oxygen concentrations decreased, but never so far as to be fatal (Science 2011, DOI: 10.1126/science.1199697). Nor did other nutrients, such as nitrogen or iron, appear to limit microbial growth (Environ. Sci. Technol. 2013, DOI: 10.1021/es303167p). One likely reason is that currents sloshed water around in the Gulf, mixing the plume with uncontaminated water that replenished the nutrients (Proc. Natl. Acad. Sci. USA 2012, DOI: 10.1073/pnas.1108820109). The end result was microbial persistance, and the deep-plume material was largely gone within months.
Just which microbes consumed plume material, what they digested, and how they did it is an area of intense study to understand spill ecology. Researchers measured compound concentrations and how they changed during the spill. They also took water samples and used the microbes in them to grow cultures or do single-cell genomics studies.
The spill dramatically altered the microbial population structure in the waters, says Terry C. Hazen, a professor of civil and environmental engineering at the University of Tennessee, Knoxville. He and colleagues detected 951 bacterial subfamilies in uncontaminated Gulf of Mexico water. In the plume, however, they found that the water was highly enriched in 16 hydrocarbon-digesting subfamilies. Once the hydrocarbon supply was capped and those groups began to die back a bit, then other creatures that feast on the petroleum degraders began to show a population boom, Hazen says.
There was a notable casualty. Filter-feeding organisms called sea squirts that live in the water column “were dead in droves,” says David L. Valentine, a professor of earth science at the University of California, Santa Barbara. “It’s clear that they were filtering something down deep that was affecting them,” although no one is sure what it was.
How the changes in microbial population and effects higher up the food web affected the overall ecosystem is likewise unknown. “We really lack a basic understanding of how that ecosystem operates normally or what happens when it’s perturbed,” Valentine says.

Of the gas and oil released from the well, what didn’t sink immediately or dissolve in the deep plume rose to the surface. There, volatile components in the oil evaporated to the air. At the time of the spill, one of the National Oceanic & Atmospheric Administration’s P-3 research aircraft was loaded with atmospheric monitoring equipment as part of a Southern California field study. NOAA briefly diverted the P-3 to the Gulf of Mexico, where it flew two missions about seven weeks into the spill. Some of the research vessels studying water in the Gulf also had atmospheric monitoring equipment on board.
In the air, scientists detected C3–C12 alkanes along with several benzene species and naphthalene. They were able to use those measurements to give an independent, quantitative estimate of the oil flow rate out of the well (Geophys. Res. Lett. 2011, DOI: 10.1029/2011gl046726).
The measurements also contributed to an understanding of air quality (Proc. Natl. Acad. Sci. USA 2012, DOI: 10.1073/pnas.1110052108). The organic compounds condensed in the air to form secondary organic aerosol (SOA) particles, which cause respiratory and cardiovascular problems. Other emissions included soot particles from controlled burns of surface oil and nitrogen oxides from fuel combustion and gas flaring in recovery operations. Nitrogen oxides undergo photochemical reactions with airborne organic compounds to produce health-harming ground-level ozone.
Some response workers complained of respiratory problems, but there could be many causes. The emissions measurements were taken too far away from workers to give a good indication of individual exposures, especially if workers were near freshly surfaced oil or a controlled burn, says Thomas B. Ryerson, a NOAA research chemist. But the large amounts of SOA in the air point to a need in future spills to monitor workers for SOA exposure, something that is currently not done.
Some of what Ryerson and colleagues learned and evaluated during the Macondo spill came in handy in 2012, when the Elgin gas-drilling platform in the North Sea suffered a leak. Within 24 hours of a flight over the site, scientists quantified the leak, showed that it was decreasing over time, and, most important, showed that the isotopic signature of the oil was from a relatively shallow, low-pressure reservoir. Originally, officials feared the gas was coming from a high-pressure formation, which could have produced a dangerous blowout, Ryerson says. The new information led authorities to decide that it was safe to put people back on the platform to kill the well, rather than take months to drill relief wells.

The lesson from Macondo and Elgin, Ryerson says, is that atmospheric measurements should be considered critical to spill response because their quick turnaround time and accurate volume estimates can help mobilize responses of appropriate scale—such as how much containment boom or dispersant to use and what size recovery vessels are needed. Low estimates of the Macondo spill meant that responders never had the capacity to recover as much oil as they could have, Ryerson says.
Advertisement
He proposes creating a targeted set of critical instruments that could be installed quickly on NOAA, Coast Guard, or other aircraft to provide key spill information within a few days of the start of a spill. Although it’s easy to show how the equipment would be cost-effective by enabling better and faster spill response, the federal budget climate has so far meant that NOAA has not been able to identify funding for such a project.
Of the oil that rose to the surface above the Macondo well, what didn’t evaporate to the atmosphere stayed on the water and was eventually pushed to shore. Through wave action, the oil emulsified with water to form what’s known as mousse. By the time it reached the coastline, it resembled a “really wet peanut butter material,” says John H. Pardue, a professor of civil and environmental engineering at Louisiana State University (LSU). Exposed to sun on the surface, it underwent photochemical oxidation, and microbial populations started to work on degrading it. But because the lighter, easier-to-digest hydrocarbon compounds had already been left behind in the deep plume or had evaporated to the air, the surface oil was overall harder to degrade.
OIL ANALYSIS
Next-Generation Techniques Identify More Than Just Nonpolar, Lightweight Compounds
When it comes to oil analysis, scientists are stuck in the 1980s—the era of early cordless phone technology rather than iPhones, says Woods Hole Oceanographic Institution scientist Christopher M. Reddy.
The key analytical tool used today is gas chromatography (GC), which primarily separates compounds on the basis of boiling points. Using GC with a flame ionization detector, oil analysts can essentially count carbons. Coupling GC with mass spectrometry (MS) gives more detail on compound identity.
GC methods are robust and have gone through fastidious quality control, Reddy says. “You can get data from five or 10 different labs across the country and be assured that there’s no variability.”
But when it comes to fingerprinting the origin of an oil sample or understanding how its composition changes as it weathers, those techniques do not paint a complete picture. GC is best suited to study only the nonpolar, lighter compounds in oil, up to about C44, and it doesn’t resolve isomers. That means GC can only identify about 25% by mass of crude oil content, depending on the specific oil, says Mark J. Benotti, a Massachusetts-based environmental chemist with the nonprofit research institution Battelle.
Researchers also point to thin-layer chromatography as a useful oil analysis technique that’s helpful for classifying compounds into saturated hydrocarbons, aromatics, oxygenated species, or heavy and complex asphaltenes. This is particularly useful for weathered samples, which tend to be highly oxygenated.
Newer technology for oil analysis includes two-dimensional GC, in which material separated on one column by boiling point is then injected into a second that further separates compounds by polarity. This approach offers better resolution than conventional GC, making it possible to identify more than 50% of compounds.
And GC with pulsed-flame photometric detection can pick up nitrogen or sulfur rather than just carbon in compounds. “The distribution of peaks gives you a sulfur fingerprint of the oil,” Benotti says.
Isotope-ratio MS, in which isotopes are compared for individual compounds, can also be used for oil-spill forensics.
Nevertheless, many compounds in oil are not amenable to GC because they’re too large, polar, sticky, or thermally unstable. Half of Macondo oil in Gulf of Mexico sediments is outside the window of even the best GC instruments, Reddy says. “That’s where liquid chromatography and perhaps spectroscopic techniques are really going to open up the field.”
And for truly exhaustive peak identification, there is ultra-high-resolution Fourier transform ion cyclotron resonance MS (FTICR-MS), a specialty of the National High Magnetic Field Laboratory at Florida State University. With the help of 9- and 14-tesla magnets, FTICR-MS can distinguish ions that differ in mass by less than an electron. Using FTICR-MS to study Macondo oil turns up more than 23,000 compounds, said Amy M. McKenna, a staff scientist at the magnet lab, in a talk in the Division of Analytical Chemistry at the American Chemical Society national meeting in New Orleans in April.
The oil industry already regularly uses advanced analytical techniques to better understand what it’s extracting and refining, Reddy says. He’s hopeful that work done by his lab and others to address the Macondo spill will accelerate their application to oil in the environment.
Wind and waves break slicks into patches, which typically show up on beaches as so-called surface residue balls or patties of oil mixed with sand. Although beaches were cleaned of such material after the spill, the oily aggregates may still turn up on Gulf of Mexico beaches when oil in underwater sediments gets stirred up from storms such as hurricanes.
Comparative analysis of the wellhead oil sample, a surface slick several weeks into the spill, and oil-sand aggregates and rock scrapings a year later by WHOI postdoctoral researcher Christoph Aeppli, along with Reddy, UCSB’s Valentine, and other colleagues showed that as the oil weathers it becomes more and more oxygenated (Environ. Sci. Technol. 2012, DOI: 10.1021/es3015138). The researchers particularly identified increases in O–H, C=O, and C–O structures and also found C10–C32 carboxylic acid and alcohol degradation products that were not present initially in Macondo oil. Such oxygenated compounds have historically not been investigated after oil spills, but clearly they are important for understanding how oil degrades. They also may be important for understanding toxicity. Much oil toxicity research focuses on polycyclic aromatic hydrocarbons, but some studies suggest that other species must also play a role.
Other researchers are looking at factors that influence microbial degradation of surface residue material, with an eye toward finding ways to improve bioremediation. Water seems to play an important role, Pardue says. A few days after being saturated with water, microbial activity kicks up, and then a few days later it dies down again. Pardue believes that the water delivers oxygen and other critical nutrients into the oil-sand aggregates.
The Gulf Coast includes some very ecologically sensitive marshes, and during the spill people feared the marshes would be decimated by oil. Although the oil did kill some grasses, plants by and large survived the spill reasonably well, says Edward B. Overton, a professor emeritus of environmental sciences at LSU. “As long as the oil is not on leafy surfaces and doesn’t affect carbon dioxide, oxygen, and water exchange, the plants seem to be fairly tolerant,” he says.
But creatures living among the plants are not necessarily as resilient. Linda Hooper-Bui, a professor of entomology at LSU, has been studying insects in the marshes. Insects serve as food for frogs, fish, and birds, so they are critical to the food web. “Insects are really great indicators of the health of the marsh,” Hooper-Bui says.
The September immediately after the spill, she and colleagues found dramatic decreases in 100 species of marsh insects, even in marsh interiors where grasses were healthy. Additional study in collaboration with R. Eugene Turner, a professor of oceanography and coastal sciences at LSU, appears to point the finger at aromatic compounds in oil, particularly naphthalene and methylnaphthalene. Their concentrations in marsh sediments have been increasing rather than decreasing, Hooper-Bui says, possibly because of degradation processes. The volatility of the compounds means that toxic effects could extend to insects not in contact with the oil itself. It could also be the reason behind reports that bird eggs in the area have been shattering easily, Hooper-Bui says.
As at the beaches, storms can raise oil out of underwater sediments and into the marshes, possibly prolonging its effect on insects. Hooper-Bui has seen insect effects on beaches, too. The oil seems to have precipitated a decline there in native ant species and an increase in invasive ones. The longer term effects of food web and species disruption remain to be seen.
Farther out in the Gulf, the oil in deeper sediments is an ongoing concern. It is also the biggest mystery and is in the hardest area to study. Oil combined with particles from the Mississippi River, and biomatter rained down on the seafloor ecosystem in a “toxic blizzard” that led to a complete die-off of plant life at the bottom of the Gulf, says David Hollander, a professor of marine science at the University of South Florida (USF). Normally the sedimentation rate is about 1 mm per year, but the spill added an additional 30–40 mm. Toxicity aside, the sediment blizzard may have simply smothered some animals.
As with surface oil, oil-based compounds in sediments, especially polycyclic aromatic hydrocarbons, tend to resist degradation. They’re also in a colder, more nutrient-limited environment with slower microbial activity. Hollander adds that the sedimentary material was very fine-grained, which reduces oxygen concentrations because it slows diffusion of oxygen from water relative to its rate of diffusion into coarser-grained material.
But that may not be the only problem. When Samantha B. Joye, a professor of marine sciences at the University of Georgia, looks at seafloor samples, she can’t detect any microbial activity in the oil-spill layer. It’s a big contrast to the vibrant microbial life she finds at natural seeps at similar Gulf depths. “That’s fascinating,” she says. “The layers are very rich in organic carbon, but it’s either so recalcitrant that it’s not being degraded or there’s something in there that is inhibiting the microbes.”
With the oil on the seafloor degrading slowly or not at all, the worry for the ecosystem is what continued exposure will do to animals. The eggs and larvae of fishes are particularly susceptible to oil contamination, even at concentrations as low as parts per billion, says Steven A. Murawski, also a professor of marine science at USF. He’s also concerned about species that burrow into sediments and is studying a species of tilefish as well as the king snake eel. Murawski emphasizes that the toxicity concerns at this point have more to do with the health of the fish than with human exposure from consuming fish. He also notes that some fish in the Gulf, such as tuna, live for 30–40 years. Population effects from exposure of their young might not appear for years.
A fuller picture of Macondo spill effects on the Gulf may yet emerge. Companies involved in the oil-spill disaster are in the middle of a trial to determine whether they acted negligently and how much oil spilled. Under the Clean Water Act, fines could be as high as $4,300 per barrel if the judge determines that the companies were grossly negligent. Many studies have been sequestered for use in the trial, and researchers look forward to their release.
“At the end of the day, this will be the most well-studied oil spill along with the biggest oil spill in history,” Murawski says. Hopefully that knowledge can be put to good use for the next spill, in the Gulf or elsewhere, to control and mitigate its impacts quickly.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter