Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
A Less Toxic Antifungal Agent
Medicinal Chemistry: Modified version of amphotericin B will drive search for effective analogs
by Stu Borman
June 17, 2013
| A version of this story appeared in
Volume 91, Issue 24

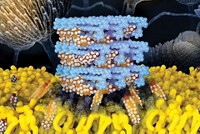
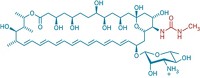
The natural product drug amphotericin B is a last-resort treatment for life-threatening fungal infections. But it can cause serious side effects, including liver damage, convulsions, and heart failure. Less-toxic versions of amphotericin B have been reported, although none have been approved for use.
University of Illinois, Urbana-Champaign, chemistry professor Martin D. Burke and coworkers are trying again. They report a modification that eliminates the drug’s toxicity in human cells, giving hope for a new class of less-toxic derivatives (J. Am. Chem. Soc. 2013, DOI: 10.1021/ja403255s).
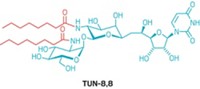
Amphotericin B is a large cyclic compound with a small appendage called mycosamine. Two years ago, Burke and coworkers showed that mycosamine binding to the fungal sterol ergosterol enables the drug to kill fungi (C&EN, March 21, 2011, page 51).
The researchers also believe that mycosamine binding to cholesterol in patients causes the drug’s side effects. They now find that deleting mycosamine’s 2'-hydroxyl group gives an analog that doesn’t bind cholesterol, retains most of the parent compound’s potency, and in lab studies eliminates side effects in human cells.
Natural product specialist Michio Murata of Osaka University calls the reduced toxicity a “surprise.” He says the derivative could be “an important lead compound for developing efficacious and safe antifungal drugs.”
Amphotericin B expert Erick Carreira of Swiss Federal Institute of Technology, Zurich, cautions that the paper’s experimental data on cholesterol interactions are subject to interpretations different from those made by Burke’s group. Carreira notes that it remains to be seen whether a derivative “generated through multistep synthetic sequences, as described in the paper, will be sufficient to displace the natural product from the clinic.”
Another amphotericin B expert, Maciej Baginski of Gdansk University of Technology, in Poland, says the study’s experiments don’t conclusively establish the 2'-hydroxyl group’s role in sterol binding. But he notes that if the new analog’s lack of toxicity “is confirmed by further studies, including in vivo animal experiments, it will open a new branch of amphotericin B derivatives that may eventually bring a new lead molecule.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter