Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Policy
Consumer Protection: House To Resume Drug Safety Push
by Britt E. Erickson
January 21, 2013
| A version of this story appeared in
Volume 91, Issue 3
The House of Representatives Energy & Commerce Committee says oversight of drug safety will be a priority again this year. Lawmakers are likely to reintroduce legislation that would establish a national system for tracking pharmaceuticals throughout the supply chain. Such language was included in an early version of the FDA Safety & Innovation Act, which authorizes the Food & Drug Administration to collect fees from the pharmaceutical and medical device industries until 2018, but it didn’t make it into the final bill that was signed into law last year.
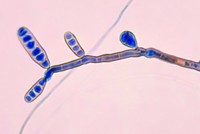
The committee will also continue its investigation of the multistate outbreak of fungal meningitis linked to the New England Compounding Center, a Massachusetts-based compounding pharmacy. Hearings on the investigation are likely, and lawmakers are expected to introduce legislation that would give FDA more authority to regulate such pharmacies. Compounding pharmacies typically mix small batches of drugs and are regulated at the state level, whereas large-scale drug manufacturers are regulated by FDA.
FDA Commissioner Margaret A. Hamburg told reporters last month that she is “guardedly optimistic” that Congress will pass legislation this year giving FDA authority to regulate compounding pharmacies. FDA’s roles and responsibilities with respect to compounding pharmacies are ambiguous under the current law, which has been subject to conflicting court opinions, she said.
House Republicans, however, are not convinced that FDA needs additional authority over compounding pharmacies. They claim that FDA had numerous opportunities to shut down the pharmacy responsible for the meningitis outbreak but failed to do so.
“In order to effectively and responsibly address the question of clarifying or enhancing FDA’s authority over compounding pharmacies, the Committee must identify what happened at the New England Compounding Center, and why FDA did not use its authority to take enforcement action against [the center] until October 2012, after the meningitis outbreak,” Republican leaders on the House Energy & Commerce Committee wrote in a Nov. 16 letter to Hamburg. The lawmakers asked FDA to provide them with all internal communications and memoranda regarding the center. “We need these documents to identify any possible weaknesses in FDA’s regulatory system that can be immediately corrected administratively or legislatively.”
MORE ON THIS STORY
- Congressional Outlook For 2013
- Chemical Regulation: TSCA Reform, Pesticide Regulations On Radar
- Energy: Tough Questions Not Likely To Be Addressed
- Homeland Security: Scrutiny Of Chemical Plant Security Program Continues
- Science Policy: Law Supporting R&D Up For Reauthorization
- Environment: Senate Looks At Climate Change; House, At EPA Science
- Taxes: Reform On Agenda In House And Senate
- Consumer Protection: House To Resume Drug Safety Push
- Trade: Congress May Be Asked To Grant White House Special Authority
- Transportation: Groups Push For Tweaks To Rail Law


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter