Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Family Of Multiply Bonded Bimetallic Complexes Grows
Series of chromium-based bimetallic complexes features a systematic variation in multiple bonding that could be useful in catalysis
by Stephen K. Ritter
September 2, 2013
| A version of this story appeared in
Volume 91, Issue 35
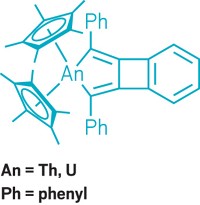
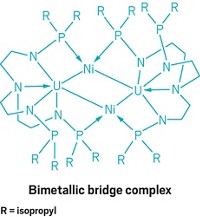
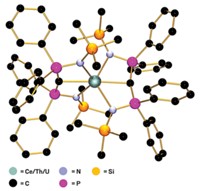
Multiple bonds between transition-metal atoms have fascinated chemists for 50 years, ever since F. Albert Cotton’s group at MIT discovered a quadruple bond in a diruthenium complex. Synthesizing these multiply bonded complexes between like metals is now common. But such complexes involving two different metals remain rare. A research team led by Connie C. Lu of the University of Minnesota, Twin Cities, has taken a systematic look at how multiple bonding varies across a series of heterobimetallic complexes in which chromium is paired with other first-row transition metals (J. Am. Chem. Soc. 2013, DOI: 10.1021/ja406506m). Chromium is an interesting target, Lu notes, because it is one of a few elements known to engage in more than a quadruple bond. In 2005, Philip P. Power’s group at the University of California, Davis, reported the first metal-metal quintuple bond in a dichromium complex. Lu’s team prepared Mn-Cr, Fe-Cr, Co-Cr, and Ni-Cr complexes that contain metal-metal bonds with bond orders ranging from one to five (two shown). The researchers’ electrochemical studies show that each complex undergoes several one-electron transfer processes. Thus, the heterobimetallic compounds could be useful in multielectron catalysis without requiring expensive precious metals.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter