Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Key HIV Entry Point Analyzed
Structural Biology: Long-sought structure of CCR5 cell receptor could improve drugs that block the virus
by Stu Borman
September 16, 2013
| A version of this story appeared in
Volume 91, Issue 37

For the first time, the structure of the receptor that is the predominant entry point for the AIDS virus to invade human cells has been determined. Knowing the form of the receptor, called CCR5, raises hopes for the discovery of drugs that can block human immunodeficiency virus more effectively than can the drug maraviroc.
When HIV infects someone, it first interacts with CD4 receptors on immune-cell surfaces. This interaction opens the door for a secondary interaction with one of two coreceptors, CXCR4 or CCR5, which results in HIV’s cell entry.
Of the two coreceptors, CCR5 is the more common entry point, and natural variations in CCR5 receptors can change a person’s risk of being infected by HIV. The crystal structure of CXCR4 bound to an antagonist (blocking agent) was obtained three years ago by Raymond C. Stevens of Scripps Research Institute California and coworkers, including postdoc Beili Wu. However, CCR5 proved more difficult to crystallize and thus to analyze structurally.
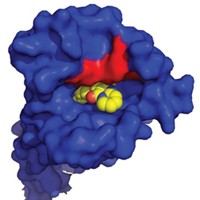
A group led by Wu, now at Shanghai Institute of Materia Medica of the Chinese Academy of Sciences, has put the second HIV coreceptor notch in its belt by determining the crystal structure of CCR5 (Science 2013, DOI: 10.1126/science.1241475). Wu and her team got CCR5 ready for its 2.7-Å (atomic-resolution) crystallographic portrait by genetically engineering a stabilized form of the receptor and steadying it further by letting the maraviroc antagonist nestle into its binding site on the protein.
The crystal structure reveals exactly where that binding site is located and how the drug orients itself to exert its activity—blocking HIV’s gp120 surface glycoprotein from binding CCR5. The study reveals that maraviroc binds at a site on the receptor different from proposed recognition sites of HIV, so the drug acts allosterically—that is, its binding causes structural changes in CCR5 that block HIV access at another site on the receptor.
Medicinal chemist Anandan Palani of Merck Research Laboratory, in Kenilworth, N.J., who helped develop another CCR5 antagonist that didn’t make it through clinical trials, calls the new study “a remarkable achievement—the solution of a long-sought problem that is potentially of great significance for designing new CCR5 antagonists with improved potency and selectivity.”
The CCR5 structure “should make it possible to rationally design molecules that block HIV efficiently with low toxicity and improved allosteric effects,” adds HIV coreceptor expert Bernard Lagane of the Pasteur Institute, in Paris. Improved allosteric effects could make it possible for drug-bound CCR5 to discriminate between viral ligands and endogenous ones “to preserve as much as possible the physiologic function of the coreceptor” and thus minimize side effects, Lagane says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter