Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Sharper View Of HIV
Biochemistry: Structural details of virus’s surface protein complex could lead to an effective vaccine
by Stu Borman
November 1, 2013
| A version of this story appeared in
Volume 91, Issue 44

The frustrating search for a vaccine effective against HIV has spanned decades and cost many millions of research dollars. Nothing produced has succeeded in halting one of the worst viral diseases ever to afflict humankind.
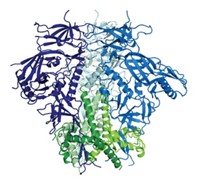
But new hope may be on the horizon. Scientists have succeeded in taking a highly detailed snapshot of HIV’s outer surface glycoprotein complex, known as Env, which punctures cell surfaces to begin the infection process. Tweaking vaccine candidates to interact more effectively with Env will be easier with more precise structural details.
The work is a landmark achievement and “represents a turning point for the field,” comments HIV-entry-inhibitor expert Carole A. Bewley of the National Institute of Diabetes & Digestive & Kidney Diseases.
Crystal structures of individual Env subcomponents and low-resolution cryogenic electron microscopy pictures have been taken before, but no one had succeeded at nailing down the detailed structure of the entire Env complex.
The problem has been that Env has so many flexible parts that it fell apart when scientists tried to crystallize it for structural analysis.
In the new work, Ian A. Wilson, Jean-Philippe Julien, and Andrew B. Ward of Scripps Research Institute California; John P. Moore of Weill Cornell Medical College of Cornell University; and coworkers reported on Oct. 31 having surmounted that obstacle (Science 2013, DOI: 10.1126/science.1245625). By modifying Env to make it water soluble while preserving its antibody-binding properties, they were able to crystallize it, enabling analysis.
The team also collaborated with Scripps’s Dmitry Lyumkis and Bridget Carragher to determine Env’s cryoelectron microscopy structure at higher resolution than before (Science 2013, DOI: 10.1126/science.1245627).
The team’s changes—made in triplicate, once for each of Env’s three pairs of glycoprotein units—involved adding a disulfide linkage between the gp41 and gp120 subunits, changing a gp41 amino acid to stabilize the trimer structure, and deleting the part of gp41 that anchors Env in the viral membrane.
Although the modified complex differs structurally from the native structure, immunological studies indicate it is a valid stand-in for it, says Eric O. Freed, senior investigator in the HIV Drug Resistance Program at Frederick National Laboratory for Cancer Research. “The resulting protein complex behaves similarly to the wild-type complex in terms of its recognition by a set of broadly neutralizing antibodies,” Freed says. The work should be important in future HIV vaccine development, he adds.
The studies reveal new information about how gp41 looks before it pokes into host cell membranes; how gp120 glycans shield the complex from host antibodies; how gp120 recognizes CD4, the main HIV receptor on host-cell surfaces; and how gp41 and gp120 interact with one another structurally. “Many researchers will be excited to interpret their findings in the context of these new structures,” Bewley says.
A separate study demonstrates how better knowledge of viral surface structure can lead to a vaccine. Barney S. Graham and Peter D. Kwong of NIH’s Vaccine Research Center and coworkers reported, also in Science on Oct. 31, animal tests showing that a surface-modified respiratory syncytial virus induces 10 times as many protective antibodies as the most promising vaccine in clinical trials for lung infections the virus causes (DOI: 10.1126/science.1243283).



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter