Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Taking NMR And MRI To The Nanoscale
New detector is poised to extend the range of existing nuclear magnetic resonance analysis techniques
by Jyllian Kemsley
December 23, 2013
| A version of this story appeared in
Volume 91, Issue 51

COVER STORY
Spectroscopy: Taking NMR And MRI To The Nanoscale
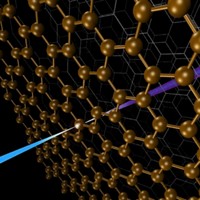
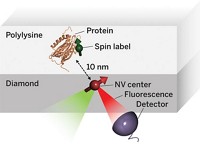
Understanding the inner workings of biology or materials science requires both structural and chemical knowledge. But current analytical techniques have their limits: They may require relatively large sample sizes, crystallization, or low temperatures, or they may reveal structural features without chemical information—or the reverse. A new approach to nuclear magnetic resonance spectroscopy and magnetic resonance imaging reported this year provides a potential way to glean both chemical and three-dimensional structural information from nanoscale-sized samples at room temperature (C&EN, Feb. 4, page 4; Science 2013, DOI: 10.1126/science.1231675 and10.1126/science.1231540). The technique was developed independently by two research groups, one led by Friedemann Reinhard of the University of Stuttgart’s Third Physics Institute, in Germany, and the other led by Daniel Rugar, manager of nanoscale studies at IBM’s Almaden Research Center, in San Jose, Calif. Rather than using an electrical coil detector, which isn’t sensitive enough to go below the microscale, the researchers used a magnetic field detector made of diamond with a crystal site defect called a single nitrogen-vacancy center. The defect involves a nitrogen atom and a crystal lattice hole that replace two adjacent carbon atoms. Prior work had determined that nitrogen-vacancy centers are sensitive to the internal magnetic fields of the diamond. The new research demonstrated that the fluorescence of such centers can be used to detect magnetic fields emanating from a nanoscale sample on the surface of the diamond detector. Both groups were able to use nitrogen-vacancy centers to home in on a signal from hydrogen atoms in a nanosized amount of poly(methyl methacrylate). Although more work needs to be done to convert the signal to the sought-after structural and chemical information, the researchers envision that the technique could be used to determine structures of small amounts of proteins that can’t be crystallized or even of biomolecules in living cells, as well as the identity and position of single atoms in nanoscale transistors in next-generation electronic devices.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter