Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Specialty Chemicals
No Clear Winner In Race To Find Non-BPA Can Linings
Chemical firms cook up new recipes, but none will work for all foods and beverages
by Melody M. Bomgardner
February 11, 2013
| A version of this story appeared in
Volume 91, Issue 6
In late January, California proposed, for the second time, to list bisphenol A as a cause of reproductive toxicity under a state law called Proposition 65. Although the maximum allowable dose would be too high to require warning labels on most products, such as food cans that are lined with BPA-based epoxy resins, the proposal adds another reason that people might want to avoid the chemical.
In the past decade, consumers and health experts have raised concerns about the use of BPA in food packaging. The molecule has a shape similar to estrogen’s and thus may act as an endocrine disrupter. The chemical industry and makers of metal food packaging contend that BPA is safe.
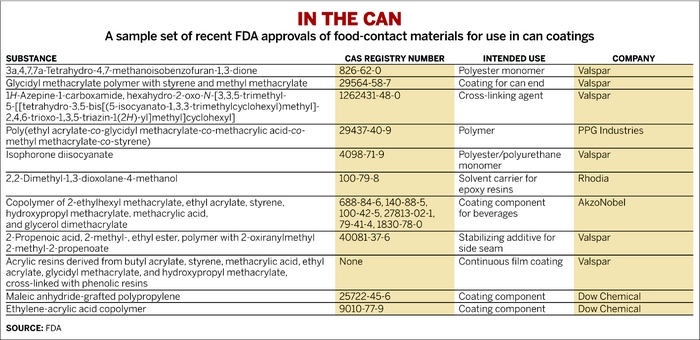
But for food companies, pleasing consumers is a high priority, and most are eager to move away from packaging based on BPA. Coating manufacturers and their suppliers are working overtime to find a replacement for the ubiquitous epoxies, which are made by reacting BPA with epichlorohydrin. A review of patent filings and regulatory approvals shows that dozens of substances are in the pipeline. They are being developed by paint firms including Valspar, PPG Industries, and AkzoNobel, and by chemical firms such as Eastman Chemical, Cytec Industries, and Dow Chemical.
The winning recipe or recipes need to meet high-performance requirements, because can coatings do double duty under difficult conditions. They protect the integrity of the can from effects of the food and protect the food from the steel or aluminum of the can.
Coatings must maintain an airtight seal, even under the high heat and pressure built up during sterilization. They must not chip, flake, or peel during handling—even if a can gets dinged. They must have minimal cost and avoid health and environmental impacts. The coating should not alter the taste or odor of the food. The most difficult hurdle for a can coating is working across different types of food and as a drop-in material in high-speed manufacturing lines.
Companies working on replacements for BPA-based epoxies are not keen to talk about product development efforts in this sensitive area. However, Jonathan Mason, associate R&D director at Dow, agreed to answer questions by e-mail. He summed up the challenge facing the industry this way: “The lowest-price, best-performing solution today is epoxy.” In addition, he predicted that no one formulation in the initial group of alternatives will work across all food and beverage types. Instead, a variety of new technologies will be required.
This viewpoint is echoed by outside experts who have evaluated alternative substances. “We could replace all BPA coatings today—but how much are consumers willing to pay, and what inconvenience will they accept?” asks Daniel F. Schmidt, associate professor of plastics engineering at the University of Massachusetts, Lowell. If lower-performing coatings are used, they may result in corrosion, shorter shelf life, and food poisoning. At the same time, Schmidt takes seriously concerns about the effect of endocrine-like chemicals on the body. “I personally think it does make sense to get rid of BPA in food cans,” he says.
Synthetic candidates to replace BPA-containing epoxies in can coatings fall largely into two main chemical categories: acrylics and polyester resins. But these base compounds can be blended with myriad other chemicals such as vinyls, urethanes, or specialty additives. And blends have been proposed that combine polyesters and acrylics; polyesters and urethanes; and even acrylics, vinyls, and polyesters. Plant-derived oleoresins have been used with low-acid foods such as beans. Making a durable coating also requires use of a cross-linker, which is another area of study.
Although most of the synthetic replacements are recipes using well-known raw materials, Eastman is proposing coatings based on a new monomer. TMCD, or 2,2,4,4-tetramethyl-1,3-cyclobutanediol, is an ingredient in Eastman’s Tritan copolyester, which has been used to replace polycarbonate, another BPA-based polymer.
None of these alternatives gets high marks in all categories of performance and safety. A recent review of can coatings for food and beverages illustrates why. For example, acrylics used in food-contact applications are brittle, and the common acrylic monomer ethyl acrylate has a noticeable odor in low quantities. Oleoresins don’t adhere well to metal substrates and do not resist corrosion when used with chemically reactive foods such as tomatoes. Polyesters can also fail when attacked by acidic foods (Int. J. Technol. Policy Manage., DOI: 10.1504/ijtpm.2013.050999).
Dow’s focus is on a coating that will prevent corrosion from high-acid foods. The company is working on a drop-in replacement for can manufacturers based on polyolefins applied as a liquid solution. “Polyolefins have been used in rigid, film, and flexible food packaging for over 30 years and have been shown to be both safe and effective,” Mason points out.
Chemical and coating companies know that any substitute they propose will be carefully scrutinized by watchdog groups. Schmidt, the plastics engineer, points out that phenolic compounds like those used to cross-link resins may also be implicated as endocrine disrupters. In addition, consumers wary of BPA are not likely to embrace vinyl-based replacements. And any compound similar to BPA, such as bisphenol S, will also be considered risky.
Schmidt, who like Eastman researchers is working to develop a can coating based on TMCD, says chemists’ hard work has resulted in some incremental improvements. “But I would be willing to bet that all of these alternatives are either lower performing, more expensive, or both, versus the usual BPA-based epoxies,” he says.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter