Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
Inside The Race To Replace Nylon 12
The auto industry and its polymer suppliers averted a crisis when a key nylon intermediate was in short supply
by Alexander H. Tullo
February 18, 2013
| A version of this story appeared in
Volume 91, Issue 7

When a cyclododecatriene (CDT) plant at an Evonik Industries chemical complex in Germany exploded on March 31, 2012, killing two workers, it didn’t take long for auto executives in far-off Detroit to realize that the tragedy could block a vulnerable choke point in their supply network.
They knew that the plant was the major supplier of CDT to facilities that make nylon 12, a substance used to fabricate brake and gas lines. Such plants are run by Evonik itself and France’s Arkema, which produce the specialty polymer via the intermediate laurolactam. Without nylon 12, production of vehicles would grind to a halt, one model at a time, in only a few weeks.
This was not welcome news to an industry that had just weathered the Great Recession and supply chain disruptions from the Japanese tsunami a year before. TI Automotive, a major supplier of brake and fuel lines, sounded the alarm. “There was a very strong likelihood at the time that a big portion of this industry would shut down,” recalls Jay Phillion, TI Automotive’s chief quality and purchasing officer.
TI Automotive did something unprecedented. With the help of the Automotive Industry Action Group, an organization that coordinates technical efforts, the firm convened a special meeting in Troy, Mich., on April 17, 2012, to avert an industry crisis. About 200 purchasing and technical representatives from car companies, part suppliers, and raw material makers attended. They took a detailed inventory of how much nylon 12 they could scare up—even scouring trucks and loading docks—and how long it would last. “When you are scraping the bottom of the barrel, you really have to look at every possible place you can get this material,” Phillion says.
Just as important, the delegates discussed how alternative materials could be fast-tracked through the auto industry’s stringent approval process. Among other tests, carmakers expose parts to fuel for 5,000 hours, see how they resist battery acid, and test for burst strength, says Jeffrey McCoy, marketing and business development manager for engineering plastics at A. Schulman, a specialty plastics maker. Fuel and brake lines, he notes, are “high liability” areas for carmakers. No one wants a recall. No one wants a lawsuit.
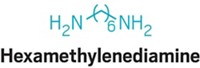
The shortage drew attention to long-chain nylon, one of the more exotic outposts of the polymers industry. The public is familiar with the term nylon because of nylon’s use in carpet, stockings and other fabrics, and films for food packaging and party balloons. Such nylon is generally nylon 6 or nylon 6,6. The numbering system describes the number of carbons in the monomers that make nylon. For example, nylon 6 is made from six-carbon caprolactam; nylon 6,6 combines hexamethylenediamine with adipic acid, both six-carbon molecules.
But nylon, or polyamide, is a broad family of polymers made via the condensation of amino groups with carboxylic acids. The long-chain nylons are derived from higher-molecular-weight monomers. A few, such as sebacic acid and decamethylenediamine, used in nylon 10,10, are derived from castor oil. As the number of carbon atoms in the monomers increases, certain nylon physical properties improve. Chemical resistance and flexibility increase; specific gravity decreases. The polymers also have less of a tendency to absorb water, a big drawback to nylons 6 and 6,6.
There is a caveat to these rules of thumb, notes Eric Gamache, technical service manager for Arkema’s specialty polyamides business. Nylon 11 has the edge over nylon 12 in flexibility and chemical resistance and is the preferred material in demanding applications such as the flexible hoses used in oil and gas exploration and production.

Long-chain nylon is produced at a much smaller scale than conventional nylon. Together, shipments of nylon 6 and 6,6 are about 6 million metric tons annually, according to Paul Blanchard, director of engineering plastics at the consulting group IHS Chemical. The combined market for nylons 11 and 12 is 100,000 metric tons annually. These specialty nylons sell for three or four times as much as the others, he notes.
But with the high prices comes performance. Nylon 12 sits at the top of the heap in auto fuel and brake lines, which need to be tough, light, flexible, and resistant to fuel, water, and road salt. “It really does have all the characteristics that you are looking for in a fuel line,” Phillion notes. Nylon 12 started to appear in fuel and brake lines in the 1980s as a light, flexible, and corrosion-resistant alternative to steel.
After the Evonik plant exploded, Phillion says, there was no way the industry could find an alternative to nylon 12 in every application. TI Automotive’s strategy was to replace it with nylon 6,12 as a coating for steel brake lines. It then moved precious nylon 12 to where it was truly needed in fuel lines.
Industry players note that the explosion merely exacerbated an existing problem. The auto industry’s rebound after the recession meant nylon 12 makers were already struggling to keep up with demand. Nylon 12 was also penetrating the market for the back panels of photovoltaic modules, where it was displacing more expensive fluoropolymers. “The solar panel market was like hooking a whale,” IHS’s Blanchard says.
Evonik had been trying to keep up. The company expanded laurolactam capacity in Marl, Germany, in 2010 and completed a nylon 12 expansion in Marl last year. It is also planning a new nylon 12 plant in Singapore. But the company still operates just one CDT plant.

The auto industry, worried about the tenuous nylon 12 supply chain, started exploring alternatives. Phillion says his company had been testing nylon 6,12 as far back as 2010. “The accident was the catalyst that made everybody jump, but there was a lot of work with different companies in preparation for the ongoing shortage issues,” says H. Chul Lee, global long-chain program manager at DuPont.
Even makers of nylon 12 were looking for alternatives. In 2011, Arkema purchased Hipro Polymers, which makes nylon 10,10, nylon 6,10, nylon 10,12, and nylon 6,12 at a plant near Shanghai, and Casda Biomaterials, which makes sebacic acid near Beijing.
Aurelien Paumier, Arkema’s North American business director for specialty polyamides, says the acquisitions gave the company a new biobased resin in addition to its nylon 11. They were also moves to keep up with the growing photovoltaic market. “The idea was to continue to supply strategic markets—automotive and transportation—despite the boom in that new industry,” he says. Since the deals, Arkema has tripled capacity at Hipro.
The acquisitions have given the company more options for dealing with the crisis, primarily nylon 10,12. “Polyamide 10,12 is close enough that it has been a very credible alternative,” Paumier says. And although the crisis has crimped Arkema’s ability to produce nylon 12, it hasn’t stopped it. The company has been able to secure some CDT through smaller alternative suppliers such as BASF and Invista.
Before the explosion, DuPont was also in the midst of developing nylon 12 alternatives. The results, Zytel RSLC 4000 and Zytel LC 7000, are based on its existing nylon 6,10 and 6,12 polymers. Nylons 6,10 and 6,12 are normally used when chemical resistance is needed above that of nylons 6 and 6,6, such as on the end tank of car radiators. The backbones of the new products, Lee says, have been chemically modified to give the polymers flexibility and chemical resistance properties similar to those of nylon 12.
Solvay’s engineering plastics unit introduced its nylon 6,10 in 2010 to complement its nylon 6 and 6,6 business. Interest took off after the explosion. “When the incident occurred,” says Pierre-Emmanuel Lucas, a new business director, “the market went mad.” The next day, Solvay got a call from a customer that uses nylon 12 for a small windshield wiper blade component. The firm had some inventory of nylon 12 but was afraid that it couldn’t compete with larger parts makers for additional supplies.
For automakers that have no option but nylon 12, help is on the way. Evonik restarted the CDT plant in December. The company won’t say when supplies will be restored, but industry sources expect it to happen in the coming weeks.
But nylon 12 makers may find that their customers don’t come back. “That is the $64,000 question,” IHS’s Blanchard says. “What will the suppliers of nylon 12 see when they are selling again?”
The extent to which the makers of alternative polymers will be able to hold on to their new customers depends on the application, Solvay’s Lucas says. Where alternatives were able to meet all the specifications, the auto industry will likely keep using new resins that have more security of supply and are often cheaper to boot. The new resins will have less success if they don’t truly meet all the specifications.
A. Schulman’s McCoy says he knows of many applications where alternatives, such as his company’s 6,12 compounds, were permanently specified in former nylon 12 applications. Automakers might have accelerated testing protocols, he says, but they didn’t cut corners. “After all the discussions and all of the meetings, there wasn’t much relaxation of the requirements,” he says.
In the end, it appears that the auto industry met the challenge posed by the crisis. North American vehicle production climbed 18% in 2012, according to J.D. Power & Associates. “We can all hold our heads high,” TI Automotive’s Phillion says. “We didn’t lose one vehicle of production. Not one.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter