Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Stacked RNA Base Analogs Could Be A Clue To Life’s Beginnings
Origin Of Life: Synthetic RNA bases defy expectations by self-assembling in water
by Carmen Drahl
February 15, 2013
| A version of this story appeared in
Volume 91, Issue 7

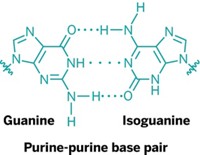
Researchers who think life on Earth may have started with a molecule like RNA face a vexing chemical problem: The bases that make up RNA can’t base-pair or assemble further in water, where life is thought to have originated. But a new report shows that small molecules reminiscent of RNA bases can indeed assemble in water—into nanofibers thousands of molecules long (J. Am. Chem. Soc., DOI: 10.1021/ja312155v).
That means it’s worth taking a new look at such intermolecular forces for origin-of-life clues, the study’s authors say.
Nicholas V. Hud’s team started creating their RNA-base-like compounds by making small tweaks to the nitrogen-rich monomers cyanuric acid and 2,4,6-triaminopyrimidine. Chemists have used these heterocycles for more than 20 years to make assemblies.
The key was to make sure the monomers could form an intermediate structure with a hydrophobic surface area of at least 1 nm2. That’s the point where the hydrophobic effect kicks in, explains Hud, a professor at Georgia Institute of Technology and director of the NASA-NSF Center for Chemical Evolution. The hydrophobic effect is sufficient to make the intermediates stack atop each other to form long conglomerates, even though the monomers are only weakly held together by hydrogen bonds in water.
Hud’s molecules stacked like gangbusters, so much so that he can’t detect any of the potential intermediate by NMR. He thinks the system might also be useful for generating hydrogel materials.
The work showcases the power of hydrogen bonding and stacking to order and arrange bases—two forces that natural RNA also exploits, says James S. W. Attwater, a junior research fellow at the Medical Research Council Laboratory of Molecular Biology in Cambridge, England. But to show that this assembly is relevant to life’s history, he cautions, the team must show how information could be passed on by molecules without RNA’s phosphodiester backbone.
Steven A. Benner of the Foundation for Applied Molecular Evolution in Florida isn’t convinced Hud’s analogs are that similar to natural RNA bases. But he’d be excited to see this result repeated with nucleotide analogs, such as Hud’s heterocycles attached to a ribose sugar and phosphate. “We’re working on it,” Hud says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter