Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Turning Ideas Into Drugs
Biotech firms rely on contract manufacturers for help and guidance on the road to market for new molecules
by Business Department
March 4, 2013
| A version of this story appeared in
Volume 91, Issue 9

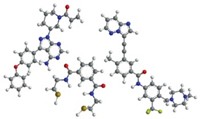
The journey of a pharmaceutical from gleam in a scientist’s eye to indispensable medicine on the pharmacy shelf is a multiyear odyssey, practically Homeric in scope, filled with victories and defeats, detours and delays, and many surprises.
COVER STORY
Turning Ideas Into Drugs
Big pharmaceutical firms usually have the tools and the skills to make the journey on their own. Smaller biotech firms aren’t so well equipped. Although they are often good at discovering molecules with the potential to become drugs, for them the road beyond that is unmapped and dark. They need help from the moment they set out.
One of the helpers biotech firms enlist is the contract manufacturer, a company skilled in chemistry and specific aspects of production that can make molecules of the quality required by regulators and in the quantity needed to test them in human clinical trials.
Here C&EN presents three stories of pharmaceutical chemical outsourcing partnerships. In all three cases, a small drug company with limited resources relies on a contract manufacturer to produce a pharmaceutical molecule for clinical trials with precision, speed, and economy.
The first case concerns Ironwood Pharmaceuticals, which discovered a peptide drug that, unlike most peptides, can be ingested by swallowing a pill rather than administered by injection. Ironwood drew on its experience in microbe engineering to create small amounts of the peptide with biotechnology. But when it needed larger quantities for clinical trials, it turned to two peptide synthesis specialists for help.
Relypsa, the biotech firm in the second case, used high-throughput experimentation to synthesize a polymer that removes excess potassium from the bodies of kidney disease patients. The technique worked well to create tiny amounts of the polymer. But the potassium binder has the potential to be a large-volume drug, so Relypsa called on an experienced custom manufacturer, Saltigo, for assistance.
The subject of the third case study, Aegerion Pharmaceuticals, was for several years a small drug company that needed help with almost everything. Aptuit, the service firm that Aegerion turned to, provided chemical manufacturing plus several other drug development services.
For Aegerion and Ironwood, the journey ended successfully last year in approval of their drugs by the Food & Drug Administration. The journey continues for Relypsa, but like the others, it is making its way with the help of able guides from the outsourcing world.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter