Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
The Turnover Fallacy
Chemists debate the best way to use reaction data to compare catalyst efficiencies
by Stephen K. Ritter
March 4, 2013
| A version of this story appeared in
Volume 91, Issue 9
\
\

\
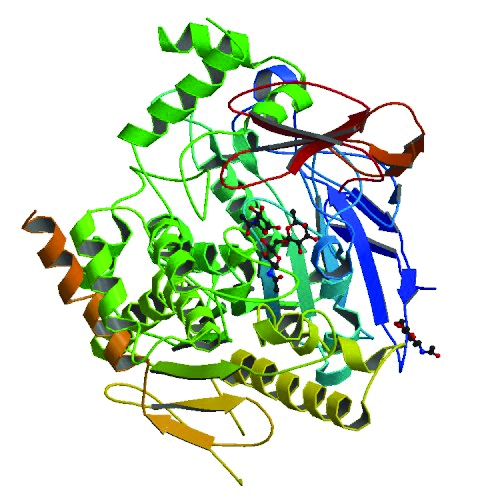
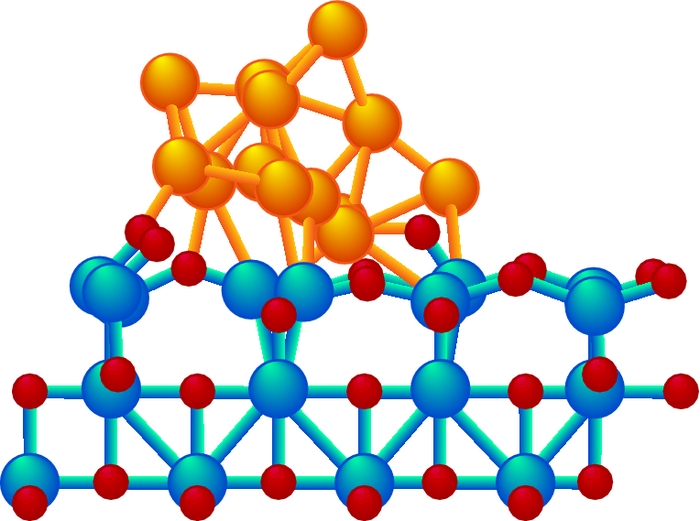
Chemists struggle with how to consistently determine efficiencies of catalysts, such as acetylcholinesterase (top), a gold cluster supported on cerium oxide (middle), and an iridium complex (bottom).
Catalysts are at the heart of most man-made and natural chemical reactions. Metal catalysts serve as the linchpin for producing many of the chemicals we use on a daily basis, such as gasoline, fertilizer, plastics, and medicines. And enzyme catalysts, which are increasingly being harnessed for chemical processing, help optimize the biochemical factories in cells that produce the energy and biomolecules required for life.
All these catalysts speed up reactions by providing alternative pathways with lower energy barriers. But when it comes to picking the best catalyst for a given reaction, chemists need a consistent way to compare the efficiency of different candidates. It’s no easy task: Metal, nonmetal, and enzyme catalysts require vastly different reaction conditions and are used on different scales. That means trying to compare different catalysts is often an apples-versus-oranges affair.
When judging the ability of individual catalysts to carry out a mission, chemists have settled on two measures: the turnover frequency and turnover number.
Turnover frequency is the amount of product formed in a catalytic reaction divided by the amount of catalyst and the reaction time. It tells researchers how fast and furious a catalyst works. Turnover frequencies are calculated in different ways, depending on how qualitative or quantitative the scientist or engineer needs to be in his or her approach.
Turnover number refers to the total number of catalytic cycles the catalyst performs during its lifetime. It provides a measure of catalyst robustness.
These metrics, coupled with reaction yields and catalyst selectivity, work well for judging individual catalysts. But chemists still lack a general way to compare different catalysts or different classes of catalysts.
Two chemists are calling for a rethink of these commonly used measures. In a viewpoint paper and follow-up discussion in the journal ACS Catalysis, theoretical chemists Sebastian Kozuch and Jan M. L. Martin of Weizmann Institute of Science, in Israel, review the misconceptions and pitfalls of using turnover frequencies and turnover numbers. They propose redefining the two measures to provide a better way to judge catalytic efficiency (DOI: 10.1021/cs3005264 and 10.1021/cs4000415).
Kozuch and Martin say they hoped their proposal would stimulate further discussion in the community—and it has. Chemists responding to the new proposal agree the current turnover definitions aren’t ideal. But they also say Kozuch and Martin’s paper gets the debate off on the wrong foot, because it assumes that a standard measure can actually be achieved. In reality, a standard measure of catalyst efficiency may be experimentally impossible.
The problems with defining a standard measure of catalyst efficiency can be highlighted by considering two main types of catalytic reactions. In high-volume chemical production industries such as petroleum refining, reactions typically take place as gaseous reactants flow across or through a fixed bed of a solid, or heterogeneous, catalyst at high temperature. The relatively low value and high volume of the products means a long-lasting catalyst with a high turnover number is often more cost-effective than a short-lived catalyst with a high turnover frequency.
For high-value specialty chemicals, such as pharmaceuticals, reactions typically take place in batch reactors using soluble, or homogeneous, catalysts at low temperatures. In this case, a short reaction time is more valuable to the manufacturer than high production volume, so high turnover frequencies are preferred and turnover numbers are less important.
Turnovers are generally useful for assessing the efficiency of a single catalyst or enzyme, Kozuch and Martin note, but not for comparing different catalysts or families of catalysts. They suggest it could be possible to make an apples-to-apples comparison of different catalysts by normalizing catalyst data to standardized reaction conditions.
Inspired by classical thermodynamic functions such as the Gibbs activation energy of reactions at standard conditions of temperature, pressure, and concentrations, Kozuch and Martin propose calculating standard-state turnover frequencies and turnover numbers. They set the standard conditions at 1 M concentration of reactants and products, or 100 kilopascal pressure in the case of gases, and a temperature of 273.15 K (0 °C).

In a comment responding to Kozuch and Martin’s proposal, chemical kinetics specialist Gábor Lente of the University of Debrecen, in Hungary, agrees that, despite the utility and common use of turnovers, the concept is still not well defined and often leads to confusion. However, he questions the value of using turnovers at all. Lente argues that more scientifically rigorous rate equations and rate constants, which include details about the reaction mechanism, should be used (ACS Catal., DOI: 10.1021/cs300846b).
Chemists draft rate equations on the basis of the experimentally observed kinetics of a reaction. Rate equations depend on the concentrations of the reactants and products and on several rate constants. A rate constant is a coefficient that provides the rate for a single step in a multistep reaction. It is independent of the concentrations. With a detailed rate equation, chemists can extrapolate the data for a catalyst to different concentrations and reaction conditions.

Lente emphasizes that turnover frequencies are often incorrectly considered to be reaction rates or rate constants when they are determined from yield data of a single experiment rather than from detailed kinetics studies. This is a problem, particularly in homogeneous catalysis, because the turnover frequency changes during a reaction as the concentrations of reactants decrease. In that regard, the turnover frequency is an average rate for the reaction, rather than the maximum rate for a catalyst.
Overall, Lente thinks Kozuch and Martin accurately describe the reservations kineticists nurture about the concept of turnovers. He applauds their attempts to suggest ways to resolve the problems. Many of their recommendations are actually about taking more extensive kinetics measurements, most of which are exactly the ones that are necessary to establish a rate equation, he says.
“What I hope chemists can take away from these discussions is more motivation to meet higher scientific standards in their research,” Lente says. “In my view, characterization of catalyst efficiency is a problem for which traditional chemical kinetics already offers a time-tested solution. There is no need to create a new one.”
Donna G. Blackmond of California’s Scripps Research Institute, an authority on catalysis and kinetics, agrees. One problem she points out with the new proposal is that the standard concentration and temperature suggested are rarely encountered in catalytic reactions.
“Setting up an unrealistic standard state to compare catalysts sheds no light on the problem of definitions and only serves to confuse researchers further,” Blackmond says. “The need to understand each case separately—each catalyst, reaction, set of conditions—remains and will not be aided by a new definition.”
Avelino Corma, a heterogeneous catalysis expert at the Institute of Chemical Technology, in Valencia, Spain, agrees that accurate comparisons demand a full kinetic study.
But that is easier said than done, he says. In practice, when a series of catalysts have to be compared, it can become a time sink to measure enough reaction kinetics to get the full rate equation for every single reaction. It’s more realistic to determine turnover frequencies and compare the values in a relative manner, Corma says.
John F. Hartwig, a homogeneous catalysis expert at the University of California, Berkeley, suggests chemists should take Kozuch and Martin’s proposal and ensuing discussion in the spirit that everyone should remember the difference between turnover frequencies, turnover numbers, rate equations, and rate constants.
“Everybody has their own needs to consider when judging what is the best way to compare catalysts,” Hartwig says. “We just have to live with the fact that we can’t distill catalyst efficiency down to a single comparative number.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter