Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Multiblock Polymer Nanoparticles Attack Tumors
Drug Delivery: All-in-one nanocarriers evade the immune system, target tumors, and release drugs inside cancer cells
by Prachi Patel
February 28, 2013

For a nanoparticle to deliver anticancer drugs to just tumor cells, the particles must evade the body’s immune system while circulating in the bloodstream, home in on tumors, and only release its payload when it is inside a cancer cell. Unfortunately, many nanoparticles in use today do not have all of these capabilities. Researchers now report a nanocarrier made from easy-to-synthesize polymers that can perform all of those tasks (ACS Nano, DOI: 10.1021/nn4002769).
A few types of nanoparticles can combine some of the desired functions and have reached clinical trials. To get closer to the ideal nanocarrier, scientists have started using structurally complex copolymer molecules. But these copolymers are difficult to synthesize and can cause immune system reactions, says Hong Tan, a polymer scientist at Sichuan University, in Chengdu, China.
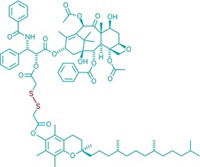
Hoping to overcome these limitations, Tan and his colleagues have designed a drug-delivery system based on long-chain polyurethane copolymers. They knew polyurethanes wouldn’t elicit an immune response because the polymers are commonly used to make medical implants such as catheters and valves. Also the materials are easy to modify and assemble, Tan says.
The nanoparticles Tan’s group made consist of polymer chains with three blocks, each capable of performing a different task. The first block contains gemini quaternary ammonium (GQA) groups, which help the particles penetrate cell membranes. The next block contains carboxyl groups to which the researchers can attach antibodies for proteins expressed by cancer cells. The chains are capped on one end with hydrophilic polyethylene glycol molecules. These groups should prolong the particles’ circulation in the body by making them less susceptible to attack from white blood cells. Each block links to the next via hydrazone groups, which can cleave under acidic conditions.
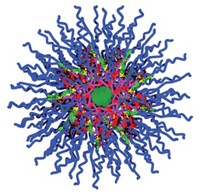
In water, the polymer chains self-assemble into spherical and cylindrical particles measuring 52 to 98 nm in diameter. Using nuclear magnetic resonance and fluorescence spectroscopy, the team determined that the particles have a hydrazone core surrounded by an inner shell of GQA and carboxyl groups, and then a shell of polyethylene glycol molecules. When the researchers add an anticancer drug to the solution, the drug gets trapped in the hydrophobic core of the polyurethane particles.
The researchers envision the particles acting like multistage rockets, shedding each shell as they move from the bloodstream to inside cancer cells. When the particles reach tumor tissue, which tends to be more acidic than healthy tissue, one set of hydrazone bonds should break, detaching the polyethylene glycol shell. The exposed carboxyl-group-linked antibodies then would anchor the particles to cancer cells and the GQA groups would help the particles tunnel into the cells. Once inside the acidic cancer cells, the hydrazone bonds in the core should rupture, breaking open the particles and releasing the drug.
To test the particles, the researchers loaded them with the cancer drug paclitaxel and injected them into mice with skin cancer tumors. Some mice received injections every three days of nanocarriers that had tumor-targeting antibodies, some got nanocarriers without the antibodies, and others just received paclitaxel alone.
Both types of particles inhibited tumor growth more than the drug alone. After 20 days, animals treated with targeted nanoparticles had tumor volumes that were one-fourth that of those receiving just drug, and one-third that of animals treated with nontargeted particles. Tan says the results suggest the particles successfully accomplished the three tasks they’d hoped.
Vladimir R. Muzykantov, a professor of pharmacology and medicine at the University of Pennsylvania, calls the multifunctional nanoparticle design elegant. “The outcomes look fairly impressive,” he adds, and suggests researchers should study further the particles’ capabilities.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter