Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Ethanol-To-Butanol Conversion A Biofuel Plus
ACS Meeting News: Catalytic process creates butanol as a better biofuel option for gasoline
by Stephen K. Ritter
April 11, 2013
Ethanol derived from corn and sugarcane has been a blessing when it comes to transportation fuels. The millions of gallons of ethanol added to gasoline in the U.S. each year help oxygenate the fuel to limit pollution and stretch petroleum supplies. One drawback is that ethanol reduces the energy content of gasoline, resulting in fewer miles per gallon when you are on the road.
Chemists and chemical engineers who are at work to find improved biofuels have decided that butanol is a better option than ethanol. The researchers are now trying to settle on the best way to mass produce butanol to start replacing ethanol.
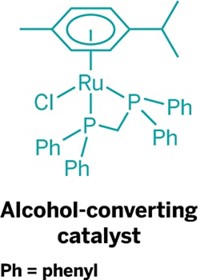
One solution offered by Duncan F. Wass and his research team at the University of Bristol, in England, is a new family of ruthenium catalysts that readily convert ethanol to butanol. Wass presented his group’s latest results during a session in the Division of Catalysis Science & Technology at the American Chemical Society national meeting this week in New Orleans.
Butanol with two additional carbons has about 30% higher energy content per gallon than ethanol, Wass explained. He noted that efforts are under way in the Midwest to retrofit some ethanol facilities to butanol production. But with his group’s new catalysts, ethanol facilities wouldn’t need to be altered—the ethanol produced in the facilities could simply be upgraded to butanol in an additional condensation reaction step.
“Our technology is an indirect path to butanol,” Wass told C&EN. “But it’s flexible because it can upgrade ethanol made from either petroleum or biomass.” The ruthenium catalysts are very efficient, he added, producing butanol with 95% selectivity and ethanol conversion of better than 40%.
The ethanol-to-butanol catalytic process is complementary to the fermentation of sugars directly into butanol using engineered microbes, which is being developed by other scientists, Wass noted. But fermentation to butanol has a few limitations, including low conversion of around 4 to 5% because of the inherent toxicity of butanol to the microorganisms.
The Bristol catalytic process has been patented, and Wass is working with scientists at BP Biofuels to develop the technology. “The new catalysts, while at an early stage, have much in common with modern petrochemical processing,” commented Ian Dobson of Butamax Advanced Biofuels, a joint venture created by BP and DuPont, and technology adviser to BP Biofuels. “The catalysts hold the prospect of being able to convert ethanol to butanol in high yield and at large scale.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter