Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
New Material For Energy-Efficient Refrigeration
Materials Science: A class of inexpensive transition-metal boride compounds could cool refrigerators via applied magnetic fields
by Katherine Bourzac
June 12, 2013

Refrigerators in our kitchens cool food by powering pumps that compress gases like Freon. Some scientists and engineers would like to scrap those energy inefficient compressors and chill refrigerators using low energy magnets. These researchers are on the hunt for practical materials that exhibit the magnetocaloric effect—the ability of a substance to heat and cool under the influence of a magnetic field. Now a team at Florida State University has shown that inexpensive transition-metal borides exhibit the magnetocaloric effect at low magnetic fields (J. Am. Chem. Soc. 2013, DOI: 10.1021/ja404107p).
A material that exhibits a magnetocaloric effect will heat up in an applied magnetic field and then cool when the field is removed. Basically, the material releases energy when a magnetic field forces its magnetic poles to align and then absorbs energy when the field is gone and the poles randomize.
In 1997, Karl A. Gschneidner Jr. and colleagues at the Ames National Laboratory demonstrated the first material with a strong magnetocaloric effect at near room temperature. Unfortunately, this alloy of gadolinium, silicon, and germanium (Gd5Si2Ge2) requires strong magnetic fields applied by an electromagnet to cool down significantly. It also contains expensive rare-earth elements.
Since that discovery, materials scientists have been hunting for magnetocaloric materials that work under the relatively weak magnetic fields produced by strong permanent magnets. Without the need to power an electromagnet, a magnetic refrigerator would require little energy to operate.
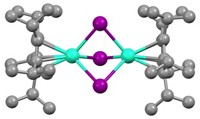
During that hunt for new materials, no one had looked at borides, says Michael Shatruk, a chemist at Florida State University. Shatruk notes that the strongest permanent magnets are made from neodymium iron boron. He thought that taking out the neodymium might give the material magnetocaloric properties. Indeed, he and his colleagues found that iron boron and manganese boron exhibit the magnetocaloric effect—but at about 300 °C. Computer modeling suggested that adding aluminum ions would bring down the temperature to more practical levels.
To test their prediction, Shatruk’s group synthesized the aluminum iron boron compound AlFe2B2 using arc melting, a high temperature process commonly used to make magnetic compounds. Next they treated the material with acid to remove impurities. Looking for a more environmentally friendly way to synthesize the material, they also developed a method to synthesize the compound in molten gallium, eliminating the need for a chemical purification step.
The material shows “a respectable magnetocaloric effect,” Shatruk says. At about 34 °C and in a 2 Tesla magnetic field, which is slightly higher than one created by the strongest permanent neodymium magnets, the material absorbed 88 J of heat energy from its surroundings per kilogram of the compound. Gd5Si2Ge2 still has a stronger effect. It absorbs 126 J of energy per kilogram of material under the same conditions.
“Nevertheless, our work shows that systems of this kind are certainly worthy of further exploration,” Shatruk says. He points out that compared to the benchmark compound, Gd5Si2Ge2, the elements in this new compound are inexpensive.
Vitalij K. Pecharsky, who works on magnetic materials at the Ames National Laboratory, agrees that the work points to a new pool of potential magnetocaloric compounds and that focusing on abundant and inexpensive materials is an important goal. However, he says that finding practical magnetocaloric materials—especially ones with no rare earths—is very difficult. “What was easy has already been done,” he says. Pecharsky adds that predicting magnetic structure in metals is particularly tough, so chemists have to do the laborious work to make the materials and test them to understand how they will behave.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter