Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Mimicking Pollen’s Sticky Tricks
Materials Science: Researchers synthesize iron-oxide replicas of pollen particles that are sticky and magnetic
by Katherine Bourzac
November 4, 2013

Researchers at Georgia Institute of Technology have turned to flowers for inspiration in designing materials with novel properties. The team synthesized magnetic replicas of sunflower pollen grains out of iron oxide (Chem. Mater. 2013, DOI: 10.1021/cm402226w). The particles’ magnetic properties and spiky surfaces allow them to adhere to a wide range of surfaces.
Kenneth H. Sandhage, a materials scientist at Georgia Institute of Technology, and his group receive funding from the U.S. Air Force to develop bioinspired materials for sensors and other applications. Sandhage recently has focused on materials like pollen that can stick to surfaces due to their shapes. “Pollens have evolved to adhere specifically to certain surfaces,” he says. For example, a grain of pollen from the sunflower (Helianthus annuus) is shaped like a burr. The 40-µm-diameter spheres are covered with tapering spines that get entangled in the hairs on bees’ legs. Van der Waals forces at the tips of the spikes help the grains to stay attached.
Sandhage’s team wanted to synthesize sticky particles by mimicking the shape of sunflower pollen and using magnetic materials. To do so, the researchers first washed sunflower pollen particles with chloroform, methanol, hydrochloric acid, and then deionized water, to remove minerals on the surface and expose hydroxyl groups on the particles’ surfaces. They coated the pollen with iron oxide using a sol-gel process: For 30 cycles, the scientists treated the particles with an iron(III) isopropoxide solution and then rinsed the surface with water to regenerate the hydroxyl groups.
Firing the coated particles in a furnace burned away the organic material at the core, leaving behind hematite (α-Fe2O3), which is weakly magnetic. Another heat treatment boosted the particles’ oxygen content and converted the hematite to magnetite (Fe3O4), which is strongly magnetic.
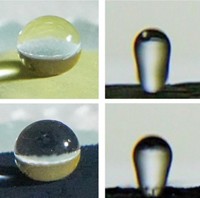
The final particles are about the same size and spiny shape as their pollen precursors. To compare the particles’ properties to those of natural pollen grains, the researchers placed both particle types on the tip of an atomic force microscope and dragged them along various surfaces, including silicon, plastics, and nickel foil. The team found that the adhesion forces in the hematite and magnetite particles were similar to those created by the washed organic pollen. The researchers also measured the magnetic properties of the particles. While the synthetic particles’ adhesive forces weaken at distances greater than about 10 nm, the magnetic forces can attract surfaces about a millimeter away.
The Georgia Tech group is currently studying pollen from other plants and exploring other metal oxides.
Using the pollen grains as a substrate was a great idea, says Simon R. Hall, a chemist at the University of Bristol, in the U.K. “To produce a structure that complex synthetically in the lab would be extremely difficult.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter