Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Compact Reactor Creates Syngas
Fossil Fuels: A simple, small-scale reformer could convert natural gas into syngas at hydraulic fracture sites
by Alexander Hellemans
December 4, 2013

Hydraulic fracturing, or fracking, has opened up myriad new sources of natural gas around the world. Not only used for fuel, natural gas is an important starting material for syngas, a combination of hydrogen and carbon monoxide that’s a feedstock for the production of diesel fuel, methanol, and other chemicals. However, due to the relatively small stream of natural gas that comes out of fracked sites, it’s usually not cost-effective to build the large steam reformers that normally turn it into syngas. Now researchers in Russia have developed a new, simple type of reformer suitable for small-scale syngas production (Ind. Eng. Chem. Res. 2013, DOI: 10.1021/ie4022489).

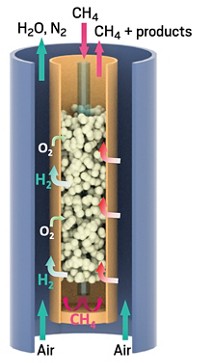
In steam reforming, methane is exposed to a nickel catalyst and steam at a high temperature. The steam reacts with the methane, forming H2 and CO. The steam reformers are difficult to scale down in a cost-effective way because they need an external heat supply to make the steam.
Vladimir S. Arutyunov of the Russian Academy of Sciences, in Moscow, and his colleagues found a way to avoid the external heater problem. They assembled a burner out of porous ceramic plates, similar to ones used in some gas-fueled outdoor heaters, arranging them as the bottom and sides of a box. A mixture of natural gas and oxygen flows through the plates’ pores into the box. When the mixture is ignited, it burns above the surface of the plates in a process called flameless combustion: The plates get red-hot, but the combustion produces no flame. Complete combustion of the methane would produce carbon dioxide and water. But by limiting the amount of oxygen in the mixture to about 40% of the level required for complete combustion, the researchers ensure that methane gets converted into H2 and CO.
Unlike in some types of steam reformers, the new burner doesn’t produce soot, which would have to be removed from the syngas before it could be used as a feedstock for making other chemicals. The combustion takes place too quickly for soot to form, Arutyunov says. The team also increased the efficiency of the reformer by using the heat of the produced syngas, which is about 1,000 °C, to preheat the methane-oxygen mixture. Preheating reduces the amount of oxygen needed in the combustion reaction.
“We believe that for low-scale production of syngas our approach will be cheaper than existing methods,” Arutyunov says. “We don’t need steam, high temperatures, or catalysts.” He and his colleagues also think their reformers will have long lifetimes, because they did not notice any degradation of the burner materials after operating their experimental unit for several dozens of hours.
Subir Roychoudhury, vice president of research and development at Precision Combustion Inc., an energy technology company in North Haven, Conn., says that small-scale steam reformers have recently been developed, but the design of the Russian team’s reactor minimizes the heat loss from the thermal process by trapping the radiation and feeding it back, making the process more efficient.
However, the reactor has one problem that is difficult to fix: The syngas contains about 6% of the incoming methane. “Ideally, if you want to make syngas, the methane ‘slip’ should be much less than 1%,” Roychoudhury says.
Industry has expressed interest in the new reformer design, Arutyunov says. His team has built a demonstration unit that can process 8 m3 of natural gas per hour.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter