Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Study Finds Variability In The Accuracy Of Methods Used To Measure Emerging Contaminants
Environmental Analysis: For chemicals from pharmaceuticals or personal care products, analysis methods may lead to inaccurate measurements or data that are not comparable between labs
by Melissae Fellet
December 20, 2013
Chemicals from pharmaceuticals and personal care products can travel from the plumbing in people’s homes through municipal water treatment plants and into surface waters. Environmental scientists worry about how much of these compounds land in the environment, because some may disrupt hormone signaling in aquatic life and in people.
But unlike with regulated contaminants, there are no standard methods for the analysis of these compounds in environmental samples. A group of researchers wanted to compare a range of techniques currently used to detect the chemicals to determine the accuracy of those measurements (Anal. Chem. 2013, DOI: 10.1021/ac403274a). They found that for some of the contaminants, the accuracy of analytical methods varied widely.
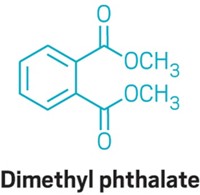
For the study, Brett J. Vanderford, a research chemist at the Southern Nevada Water Authority, and his colleagues asked 25 research and commercial laboratories in the U.S., Canada, Europe, and Australia to test water samples. The researchers spiked water with known concentrations of different collections of contaminants. They selected 22 compounds to analyze, including the antibacterial agent triclosan, the plastic ingredient bisphenol A, and the semisynthetic hormone 17α-ethynylestradiol. Three different types of water were used in the study: deionized water, drinking water from a treatment plant in Henderson, Nev., and surface water from a bay in Lake Mead, Nev.
Vanderford and his team asked the labs to determine what was in each sample and at what concentration. They told the labs to use whatever methods they pre-reported for environmental analyses. In the end, Vanderford’s group received contaminant concentrations measured with 52 different methods that varied in protocol, calibration, sample preparation, and instrumentation.
The researchers determined the accuracy of the measurements by calculating the percent bias, which represents the difference between the measured concentration and the known amount placed in the sample. For most of the compounds in drinking water, the median bias across the different techniques fell between 15 and 20%. But for some compounds, the range of biases was quite large, indicating that the accuracy of the techniques varied greatly. For example, the anticonvulsant carbamazepine had biases ranging from 1.5 to 2,000% in drinking water.
Other compounds, like bisphenol A, ciprofloxacin, 4-nonylphenol, and 4-tert-octylphenol had a narrow bias range, but the median biases were high—35 to 51% in drinking water and 38 to 59% in surface water. The high biases indicate that those compounds are generally more difficult to analyze, Vanderford says.
Bisphenol-A, 4-nonylphenol, and 4-tert-octylphenol were also prone to detection even if the compounds were not placed in the original water samples. The false positive rates for these compounds were greater than 15%, more than three times greater than the majority of the compounds on the list.
Vanderford says the results reveal the importance of developing standardized analytical methods for measuring contaminants. Based on the findings, the researchers recommend using liquid chromatography-tandem mass spectrometry for analysis combined with isotope dilution for calibration. This combination of methods produced less than 10% bias for most of the 22 compounds studied.
P. Lee Ferguson, an environmental analytical chemist at Duke University, says the field needed this study. Without standard methods, labs were performing analyses as best they could. But data from this paper indicate that the measurements are inherently challenging, even for experienced environmental chemists.
Edward T. Furlong, a chemist at the United States Geological Survey, hopes analytical chemists can use the data from this paper to provide benchmarks for new methods. “I think we all want good data,” he says, especially in cases in which the contaminants are at low concentrations and the chances of a false positive or false negative are high.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter