Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Nanostrings Of Palladium And Gold
Researchers link metal nanorods of different types and sizes end-to-end, giving the chains tunable optical properties
by Lauren K. Wolf
March 10, 2014
| A version of this story appeared in
Volume 92, Issue 10
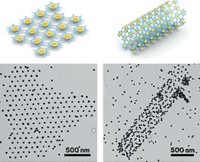
Just as scientists form polymer chains by linking molecules together, they can form so-called nanochains by stringing together various nanosized particles. Researchers think strings of metallic nanoparticles in particular might become future components on computer chips or tiny antennae that convert light into energy. A research team led by Eugenia Kumacheva of the University of Toronto has developed a way of controllably linking metal nanorods of different types and sizes end-to-end (Angew. Chem. Int. Ed. 2014, DOI: 10.1002/anie.201309718). In the past, Kumacheva’s group assembled chains of identical gold nanorods. In the new work, however, the team builds nanochains made of palladium and gold rods. Kumacheva says these mixed-metal chains have more interesting optical properties than the gold-only versions. The researchers also built “block copolymers” composed of tracts of long and short gold nanorods. To make these nanochains, the team attaches polystyrene strands to the ends of the rods. Then the researchers add a polar solvent to various mixtures of palladium and gold rods, causing the polystyrene strands to associate and forcing the rods to line up and stick together tightly.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter