Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Lactone Helps Push Carbon Dioxide To Couple With Olefins
ACS Meeting News: Chemists use palladium-catalyzed lactone formation to overcome the thermodynamic barrier to coupling carbon dioxide with dienes to make polymers
by Stephen K. Ritter
March 24, 2014
| A version of this story appeared in
Volume 92, Issue 12
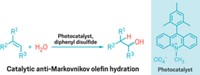
Carbon dioxide has attracted attention as an inexpensive renewable carbon feedstock to incorporate into polymers. But the low reactivity of CO2 has impeded progress in copolymerizing it with olefins. Kyoko Nozaki of the University of Tokyo described a strategy to overcome the copolymerization barrier. It hinges on cobbling together CO2 and a pair of butadiene molecules via a palladium-catalyzed reaction to form a lactone intermediate. The work builds on the Nozaki group’s palladium-catalyzed olefin copolymerization reactions and work by other groups to form lactones from CO2 and epoxides to make polycarbonates. When mixing ethylene with CO2, a thermodynamic barrier favors ethylene coupling and polyethylene formation, bypassing the less reactive CO2 and failing to achieve copolymerization, Nozaki explained. But when using a 1,3-diene, such as butadiene, she said, the palladium catalyst tips the conditions in favor of coupling CO2 and two diene molecules together. Free-radical polymerization of the resulting lactone leads to high-molecular-weight polylactones—a new class of polymers. The process also works when using two different 1,3-dienes, such as butadiene and isoprene, which leads to new terpolymers (Nat. Chem. 2014, DOI: 10.1038/nchem.1882).



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter