Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Thin Metal Organics Join 2-D Club
Transmetalation increases the members of a family of ultrathin crystals
by Mitch Jacoby
April 7, 2014
| A version of this story appeared in
Volume 92, Issue 14
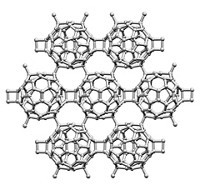
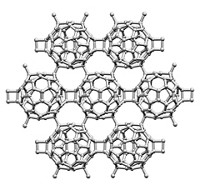
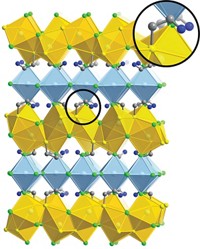
One strategy for customizing the properties of some organic polymers and metal alloys involves swapping out their constituent monomers or metal atoms. That type of exchange chemistry, which requires that the constituents be involved in active equilibrium processes, has been exploited by a multinational team led by Zhikun Zheng and A. Dieter Schlüter of ETH Zurich to make a family of two-dimensional metal-organic networks (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja501849y). The team prepared a series of ultrathin samples made up of tri- and hexafunctionalized terpyridine-based monomers joined by Zn2+ ions. The researchers then selectively exchanged the zinc ions with Fe2+, Pb2+, and Co2+. The new materials join the small but growing number of atomically (or nearly atomically) thin materials, such as graphene, boron nitride, and molybdenum disulfide, that are strong enough to be transferred to microscopy grids. These holey supports leave relatively large sections of the films consisting of millions of repeat units suspended over micrometer-sized holes. The mechanical strength of the films and their ability to be isolated and manipulated are essential to using them in molecular electronics, imaging, and other applications.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter