Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Constructing Crowded Carbons
Organic Synthesis: Chemists use Mizoroki-Heck reaction to tackle tough-to-make motif
by Bethany Halford
April 11, 2014
| A version of this story appeared in
Volume 92, Issue 15

In an advance that could help chemists build complex natural products and pharmaceuticals, researchers have found a new way to make quaternary carbon centers. The reaction is enantioselective, giving scientists a tool to make just one enantiomer of a chiral compound.
Quaternary carbon centers often present a stumbling block for chemists trying to make complex molecules. There simply aren’t many ways to create this crowded chemical motif, in which a carbon makes four bonds to other carbon atoms. Until now, to create quaternary carbons chemists had to use a functional group handle, such as a carbonyl, on the carbon adjacent to the quaternary carbon construction site. But removing that handle later can be difficult.
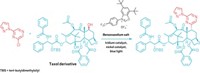
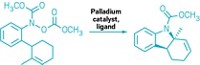
University of Utah chemists Matthew S. Sigman, Tian-Sheng Mei, and Harshkumar H. Patel found they could enantioselectively create quaternary carbons without using a nearby functional group handle. To do this, they use a palladium catalyst to perform a Mizoroki-Heck-type reaction that adds an aryl group from an aryl boronic acid to a trisubstituted alkenyl alcohol (Nature 2014, DOI: 10.1038/nature13231).
It was a fairly simple idea, Sigman tells C&EN, but the group had doubts. They wondered whether the trisubstituted alkenyl alcohol would bind well enough to the catalyst. If it did, would the aryl group add to the more substituted carbon to make the quaternary center?
The addition took place as they had hoped, and the alcohol in the substrate acts as an escape route for the palladium catalyst by undergoing oxidation. Without the alcohol, the catalyst would wander along the substrate’s alkyl chain with no way to leave.
“It is likely that this process and the building blocks prepared by this method will see great use in the synthetic community,” comments Brian M. Stoltz, an expert in organic synthesis at Caltech. A particularly useful aspect to this chemistry, Stoltz notes, is that preexisting stereocenters in the substrates aren’t affected by the reaction.
At the moment, the reaction can be used only with aryl boronic acids, but Sigman says his group is working to expand its scope so that alkyl groups can be added to the substrates as well.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter