Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Easier Route To Polyenes
Organic Synthesis: With just 12 reagents and one coupling reaction, chemists make most polyene motifs found in natural products
by Stu Borman
May 19, 2014
| A version of this story appeared in
Volume 92, Issue 20
Researchers have devised a technique in which 12 building block reagents and a single coupling reaction are used in an iterative manner to create the double-bonded core structures of most polyene natural products.
The technique moves polyene synthesis from the realm of using handwritten recipes to cook each dish from scratch in grandma’s kitchen to that of clicking Lego pieces together in the kids’ playroom.
Polyenes—alkene-based natural products such as polyterpenes, polyketides, peptide-polyketide conjugates, and fatty acids—are used as drugs, pigments, and fluorescent probes, among other applications. Chemistry professor Martin D. Burke of the University of Illinois, Urbana-Champaign, who led the team that developed the approach, says the team eventually hopes to replace current routes to polyenes with easier, more efficient, and automated ones similar to those used in peptide and DNA synthesizers.
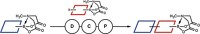
To do so, Burke and grad students Eric M. Woerly and Jahnabi Roy created bifunctional building blocks representing most of the alkene components they found in a database search of nearly 3,000 polyene natural products (Nat. Chem. 2014, DOI: 10.1038/nchem.1947).
They show that more than 75% of those compounds can be assembled by using several steps of Suzuki-Miyaura coupling to combine units from their menu of 12 N-methyliminodiacetic acid (MIDA) boronate building blocks. Suzuki-Miyaura coupling is a palladium-catalyzed reaction that combines boronate and halide organic groups. Burke and coworkers earlier discovered that the MIDA ligand greatly improves the reaction by making boronates more stable.
Among the polyenes they created in the new study are several never before assembled synthetically, such as the complex polyterpene neurosporaxanthin β-
The technique could help sidestep the need for specialists to plan and execute polyene syntheses, Burke says. It can be readily adopted by researchers because MIDA boronates are available commercially from Sigma-Aldrich, AllyChem, and BoroPharm.
Burke and coworkers “identify common building blocks that can be mixed and matched to enable synthetic access to most members of the polyene family,” comments organic chemist Paul A. Wender of Stanford University. “If I can take a word from architecture and building, a set of parts is ‘prefabricated’ and then assembled in various ways with some effective coupling chemistry. Projecting forward, this approach could indeed be automated,” he says.
Burke thinks the same strategy could be used for far more than just polyenes, which he estimates make up 1% of natural products. He believes building blocks can eventually also be developed to access most of the remaining 99%.
Wender says the prefab technique is unlikely to be effective for complex small molecules with high levels of functional group diversity. “But that takes nothing away from this approach, as where it is applicable it is a potentially powerful strategy.”





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter