Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
C–C Coupling Repertoire Grows
Organic Synthesis: Reaction eases addition of chiral carbon centers to aryl groups
by Stu Borman
June 12, 2014
| A version of this story appeared in
Volume 92, Issue 24

The revival of a nearly 50-year-old technique for forming carbon-carbon bonds may help ease the synthesis of aryl derivatives, such as drug candidates.
Varinder K. Aggarwal and coworkers at the University of Bristol, in England, have taken a venerable but neglected synthesis called Zweifel olefination and made it new again (Nat. Chem. 2014, DOI: 10.1038/nchem.1971).
The team’s approach overcomes key limitations of Suzuki-Miyaura coupling, the reaction most often used in the pharmaceutical industry for creating C–C linkages. In Suzuki-Miyaura coupling, a transition-metal catalyst is used to join sp2 carbon atoms (like those in aryl groups) to one another. It cannot easily link sp2 to sp3 (single-bonded) carbon atoms, especially in a stereocontrolled manner.
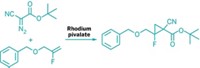
The new synthesis reported by Aggarwal and coworkers readily links sp2 carbon atoms to sp3 carbons with excellent stereocontrol and without use of transition metals. It reacts aryllithium reagents with chiral secondary or tertiary boronic esters to form aryl products with a chiral tertiary or quaternary carbon center (linked to three and four other carbons, respectively).
“It is a special paper,” says organoboron chemist Dennis G. Hall of the University of Alberta, Edmonton. “There is some precedent—there always is—but it achieves a feat that is virtually unachievable by regular Suzuki-Miyaura coupling.”
“It obviates difficulties introduced by some of the side reactions often seen in transition-metal chemistry,” comments another organoboron chemist, Cathleen Crudden of Queen’s University, in Kingston, Ontario. “The fact that it can be employed for the generation of quaternary centers with high levels of enantiomeric purity is remarkable,” she says, noting that coupling reactions of that kind have generally not been available before.
Earlier, Aggarwal’s group developed an enantioselective way to make secondary and tertiary boronic esters. “We were keen to find a way of coupling them to aromatics but recognized that this was a very difficult problem using classical Suzuki-Miyaura-based conditions,” he says. “We wondered whether we could take a leaf out of the Zweifel olefination reaction reported almost 50 years ago.” That reaction uses vinyllithium to make vinyl-alkyl products (J. Am. Chem. Soc. 1967, DOI: 10.1021/ja00990a061 and 1968, DOI: 10.1021/ja01024a068).
Aggarwal and coworkers substituted aryl for vinyl starting materials, optimized the process, and made it stereoselective. In the revived reaction, lithiated electron-rich aromatics and heteroaromatics are added to chiral secondary and tertiary boronic esters to form boronate intermediates. An electrophile such as N-bromosuccinimide is then added to induce a stereospecific rearrangement, eliminating the boronate and creating final products.
The new reaction does have drawbacks. It tolerates only a limited range of functional groups on the starting materials and requires electron-rich aromatics. Aggarwal and coworkers are currently trying to expand the reaction’s scope to a broader range of functional groups and aromatics.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter