Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
More Details On Microwave Effects
Chemists find little difference in reaction rates between microwave and conventional heating once reaction temperatures are achieved
by Stephen K. Ritter
June 16, 2014
| A version of this story appeared in
Volume 92, Issue 24
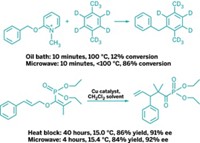
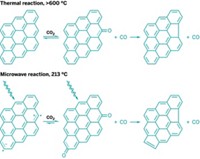
Palladium-catalyzed cross-coupling reactions have previously been reported to proceed substantially more quickly with microwave irradiation than when they are heated to the same temperature by conventional heat sources such as an oil bath. A number of researchers have investigated this phenomenon and come to a range of opinions on the exact cause. New data from Andrew W. Holland and coworkers of Idaho State University support the consensus view that microwaves induce only a thermal effect and that nonthermal radiation effects are not at play (React. Kinet. Mech. Catal. 2014, DOI: 10.1007/s11144-014-0733-z). Unsatisfied with prior explanations for faster microwave Buchwald-Hartwig aminations, Holland’s group constructed experimentally difficult kinetic profiles of reactions and found that the rate improvement achieved by microwave heating occurs mostly in the beginning of a reaction and is magnified by catalyst deactivation. The faster rates can be reproduced in an oil bath simply by mixing preheated reaction components, which mimics the short time it takes to ramp up to the reaction temperature. “Such conclusions take nothing away from the value of microwave ovens as tools in synthetic laboratories,” the researchers write. “Microwaves often remain far more convenient than alternatives—but they illustrate that the advantages of microwaves can often be enjoyed using more traditional methods.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter