Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
‘Green’ Route To Chromanols
Organic Chemistry: Organocatalysis creates tocopherol’s chiral core
by Stu Borman
July 18, 2014
| A version of this story appeared in
Volume 92, Issue 29

Researchers have developed a “green” method for creating chiral chromanols, two-ring aromatic structures that can be elaborated into bioactive compounds such as tocopherols. The antioxidant and other properties of tocopherols and related compounds make them good prospects for drug discovery, but they are difficult to synthesize.
Natural sources of tocopherols and related compounds are sparse, and catalytic methods used to produce their chiral chromanol cores are often hampered by low catalyst activity, modest enantioselectivity, toxicity, or high cost. So tocopherols are synthesized commercially as a mixture of eight stereoisomers. And no asymmetric synthesis of α-tocopherol, the most bioactive form of vitamin E, is available.
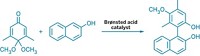
Kazuaki Ishihara and coworkers at Nagoya University, in Japan, have now devised a highly efficient, enantioselective, environmentally benign, and inexpensive organocatalytic synthesis of chromanols. The procedure uses a chiral hypoiodite oxidative organocatalyst and a peroxide cooxidant to catalyze cyclic ether formation in single-ring aromatic substrates. The resulting chiral chromanol products can be derivatized into tocopherols, tocotrienols, and other compounds (Science 2014, DOI: 10.1126/science.1254976).
The synthesis “stands out as an innovative and potentially practical strategy, in light of its high turnover, intriguing mechanism, and environmentally benign oxidative conditions,” says Hong C. Shen, head of medicinal chemistry at Roche Innovation Center Shanghai.
Biocatalysis specialist Wolf-D. Woggon of the University of Basel agrees that it’s “an excellent approach to chiral chromanols.” But he adds that elaborating chromanols to tocopherols is complex, making this route uncompetitive with an earlier organocatalytic method (Angew. Chem. Int. Ed. 2008, DOI: 10.1002/anie.200801765). Ishihara responds that not only tocopherols but also other bioactive and pharmaceutically interesting compounds “can be synthesized in short steps from our chromanol products.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter