Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Site Specific: Developers Aim To Create Well-Defined Drug Conjugates
by Ann M. Thayer
January 20, 2014
| A version of this story appeared in
Volume 92, Issue 3
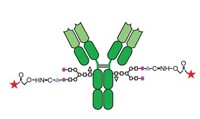
Homogeneity may be bad for creativity, but it’s good for antibody-drug conjugates. A well-defined loading—in the number and position of drug molecules conjugated to an antibody—can make an ADC easier to purify and characterize, and possibly more stable and functional as a therapy.
COVER STORY
Site Specific: Developers Aim To Create Well-Defined Drug Conjugates
Some companies are using mutagenesis or nonnatural amino acids to create linkage sites on an antibody to attach drug molecules, but another tack is to take advantage of an antibody’s natural structure.
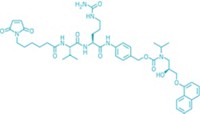
This approach is at the core of the technologies used in the two approved ADCs and many of those in clinical development. A method from ImmunoGen attaches cytotoxins to lysines in the antibody. Because there are dozens of conjugatable lysines, the result is a mix of positional isomers with an average drug-to-antibody ratio (DAR) of about 3.5.
“One of the advantages of linking through the lysine residues is that they are not necessarily integral to the structure of the antibody, and therefore it is unlikely to have a major impact on pharmacokinetics,” says Charles Morris, ImmunoGen’s chief development officer.
Alternatively, Seattle Genetics targets four interchain disulfide bridges in the antibody’s hinge region. Breaking these bridges creates eight cysteine thiols as linkage points. Although the locations are more specific than lysine, the ADC can have DARs from zero to eight, with the average controlled around four.
Two ADC technology developers, PolyTherics and Igenica, are targeting this same cysteine-rich region but with new conjugation routes. PolyTherics’s ThioBridge reagent is designed to maintain an antibody’s shape and stability by reconnecting the cysteines through a three-carbon bridge to which a toxic payload is already conjugated. The firm is partnering with payload developers to access a range of drug-linker combinations to use with its bis-thiol alkylating reagent.
Efficient and predictable, the conjugation process improves solubility and reduces aggregation of the ADC while also resulting in a narrow DAR distribution, according to PolyTherics. “We achieve very high yields, up to 90%, with a DAR of four,” says Antony Godwin, the firm’s vice president of chemistry. ADCs with ThioBridge don’t suffer from deconjugation or cross-conjugation reactions, he says.
Similarly, Igenica’s SNAP technology uses bifunctional linkers to maintain the interchain bonding. It works in conventional manufacturing processes and with most linkers and payloads.
“We have performed proof of concept in rodents and have demonstrated that our linkers are at least as efficacious if not better than the conventional linkages,” Igenica CEO Mary Haak-Frendscho says. “We suspect that as the industry gets more experience the drive will be toward homogeneous ADCs, not just to create the drug quality but the uniformity and the reproducibility of the drug product” that regulators want to see.
To date, however, none of the new approaches has been fully developed or has passed regulatory muster. Meanwhile, ImmunoGen’s Morris notes that his firm has proven that its technology works and is acceptable to regulators: It is used in the Roche cancer drug Kadcyla. The company is advancing its ADC technology through new linkers, toxins, and antibodies, but “there is still a lot to be done with the current generation of ADC technology,” Morris says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter