Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Adding Fluorine Helps Ailing Antibiotic
Tests in cell cultures show that a single fluorine helps neomycin outwit drug-resistant bacteria
by Carmen Drahl
September 22, 2014
| A version of this story appeared in
Volume 92, Issue 38
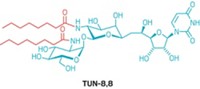
A strategically placed fluorine today could keep antibiotic resistance at bay. Aminoglycosides are a class of antibiotics that have traditionally been useful against infections in patients with severe burns or cystic fibrosis. Antibiotic-resistant bacteria have emerged, however, carrying an arsenal of aminoglycoside-inactivating enzymes. One approach to counter the enzymes has been to remove certain aminoglycoside hydroxyl groups. But that change can make the drugs toxic to the kidneys. Stephen Hanessian and colleagues at the University of Montreal instead tinkered with neomycin B, an aminoglycoside and the active ingredient in popular over-the-counter antibiotic ointments, to see whether fluorination might help. When Hanessian’s team replaced the 4´-hydroxyl group on neomycin’s A-ring with an axial fluorine (shown), they created an analog that evades aminoglycoside-inactivating enzymes in bacterial cell cultures (Chem. Sci. 2014, DOI: 10.1039/c4sc01626b). Adding an (S)-hydroxyaminobutyric acid to neomycin’s B-ring and fluorinating it was also effective (ACS Chem. Biol. 2014, DOI: 10.1021/cb5003416). To see how fluorine made a difference, Hanessian’s team used X-ray crystallography. Aminoglycosides interfere with bacterial protein synthesis by binding tightly to a microbe’s ribosomal RNA in a position called the A-site. The crystal structure shows that axial fluorine contacts a guanine nucleic acid in the A-site.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter