Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Direct N-Alkylation Of Amines
A tandem condensation-reduction process for N-alkylation of amines uses readily available carboxylic acids for the first time
by Stephen K. Ritter
September 29, 2014
| A version of this story appeared in
Volume 92, Issue 39
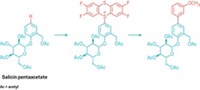
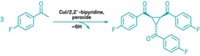
Alkylated amines are versatile building blocks used across the chemistry landscape, and over the years chemists have developed an array of synthesis methods to make them. One well-established approach is the direct reductive amination of carbonyl compounds such as aldehydes or ketones. Iván Sorribes, Kathrin Junge, and Matthias Beller of Leibniz Institute for Catalysis at the University of Rostock, in Germany, now report the first catalytic N-alkylation of amines using more stable and more readily available carboxylic acids (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja5093612). The researchers carried out the platinum-catalyzed reactions using a variety of primary and secondary amines and carboxylic acids with phenylsilane as the hydride reducing agent. The team showed the versatility of the approach by preparing fluoroalkyl-substituted anilines (one shown). The researchers also report the first one-pot catalytic synthesis of cinacalcet hydrochloride, an alkylamine-based drug for treating hyperparathyroidism associated with kidney disease and hypercalcemia associated with parathyroid cancer.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter