Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
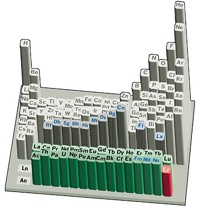
FBNCO—A Winning Combination In Elemental Bingo
Chemists have prepared a gas-phase molecule that is just a neon short of containing all the 2p-block elements
by Stephen K. Ritter
October 20, 2014
| A version of this story appeared in
Volume 92, Issue 42
Anna Troiani and Giulia de Petris of Sapienza University of Rome and their colleagues get an A for effort after mixing and matching elements to prepare the unusual molecule FBNCO. The bent-chain radical species is believed to be the first experimentally observed compound that contains one atom of each of the 2p-block elements in the periodic table, with the excusable exception of the inert noble gas neon. The team created FBNCO in a sequence of events by ionizing boron trifluoride (BF3) in the presence of isocyanic acid (HNCO) in a mass spectrometer (Chem. Commun. 2014, DOI: 10.1039/c4cc05217j). The team isolated and probed the short-lived FBNCO in the gas phase for less than a microsecond, but that was long enough to use neutralization-reionization mass spectrometry coupled with theoretical calculations to determine the connectivity of the elements and suss out the molecule’s energetic properties. The radical’s unpaired electron is centered on the boron atom, and the molecule has a dissociation energy of 50 kcal/mol and an isomerization energy of 21 kcal/mol, meeting stability criteria to make it a certifiable compound, the researchers note. The next step will be to isolate the species in solution or as a solid, in which case FBNCO potentially could play a role as a building block in nanofilms for electronics.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter