Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Chemists Find New Vein Of Fluorinases
Genome mining uncovers three versions of a rare natural enzyme that catalyzes formation of carbon-fluorine bonds
by Stephen K. Ritter
February 3, 2014
| A version of this story appeared in
Volume 92, Issue 5
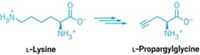
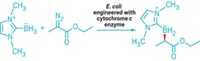
Naturally occurring organofluorine compounds are rare, with only five examples reliably known. The enzymes responsible for carbon-fluorine bond formation during the biosynthesis of those compounds are rarer still: Until now, only one has been identified. David O’Hagan of the University of St. Andrews, in Scotland, led the team that discovered the first fluorinase in 2002. O’Hagan and colleagues have now found three new versions of the enzyme (ChemBioChem 2014, DOI: 10.1002/cbic.201300732). After monitoring global databases of bacterial genomes for years, the researchers saw three fluorinase genes pop up from three genomes in the past two years. They implanted and then expressed the genes in Escherichia coli to confirm they code for fluorinases. The original fluorinase has been explored for efficiently introducing 18F into single-dose amounts of positron emission tomography imaging agents, O’Hagan notes. But limited success has been achieved in cloning the original fluorinase gene into other bacteria to produce organofluorine compounds. With the newly discovered versions, there’s more genetic information to see how different organisms handle fluorine metabolism, he says. Engineering fluorinase genes from the larger pool into other bacteria could lead to a viable fermentation technology for producing fluorinated drug candidates and fine chemicals, O’Hagan suggests.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter