Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Protein Array Guides Nanocrystal Growth
Redox protein in zinc-coordinated array serves as a template for nanofabrication
by Stu Borman
February 17, 2014
| A version of this story appeared in
Volume 92, Issue 7
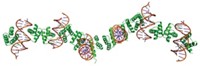
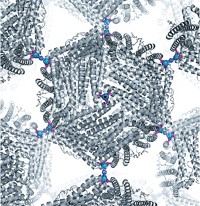
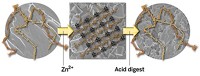
Two years ago, F. Akif Tezcan of the University of California, San Diego, and coworkers showed that an electron-transfer protein could self-assemble via zinc ion coordination into one- and two-dimensional arrays (C&EN, March 12, 2012, page 9). At the time, nanotechnologist Chengde Mao of Purdue University commented that applications of the work could include using the metal-linked protein arrays “as templates for nanofabrication.” Tezcan, Jeffrey D. Brodin, and coworkers have now demonstrated just that (Proc. Natl. Acad. Sci. USA 2014, DOI: 10.1073/pnas.1319866111). The researchers show that a cytochrome protein in a metal-based array is much more stable to heat and organic solvents than the native protein and that its light-driven redox activity can be used to control inorganic nanocrystal growth. Stable arrays of other proteins and enzymes with different functions could also potentially be used. Manuel A. Navia of Oxford Bioscience Partners, who developed cross-linked enzyme crystals, says the new technology “still has a ways to go, but I am both curious and excited about the never-before-seen polymers and other interesting materials it could eventually be used to create.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter