Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Tracking How Memories Form
Researchers watch real-time movements of memory-storage molecules inside living nerve cells
by Lauren K. Wolf
February 17, 2014
| A version of this story appeared in
Volume 92, Issue 7
When trying to understand how the human brain forms memories among its 86 billion neurons, neuroscientists typically tinker with nerve cells in a petri dish. The researchers probe these cells—isolated from lab animals—by adding fluorescently labeled reporter molecules and observing how and where inside the cells the brightly lit compounds travel.
But these molecules aren’t native to the neurons. So when they latch on to a protein or RNA strand of interest inside a nerve cell, it’s always possible that the foreign compounds are disrupting the natural flow of things.
Not satisfied with the uncertainty in this type of experiment, a research team led by Robert H. Singer at Albert Einstein College of Medicine, in the Bronx, N.Y., genetically engineered a mouse to make its own reporter molecules. The rodent produces fluorescently labeled messenger RNA (mRNAs) strands that code for β-actin, a structural protein thought to be involved in memory formation.
Using neurons and brain slices from the engineered animal, Singer and his team have been able to watch these “endogenous” mRNAs shuttle back and forth in real time along the nerve cells’ many branches. They published two papers on this technique and the dynamics they observed late last month in Science (2014, DOI: 10.1126/science.1242939 and 10.1126/science.1239200).
“When we set out to do this project five or six years ago, nobody gave us a chance of success—even remotely,” Singer recalls. That was because the gene that codes for β-actin is an essential one, he explains. If it gets altered too much, it doesn’t work correctly and the mouse dies. But amazingly, the complex adjustments Singer’s group made to get its mouse’s β-actin mRNA to light up don’t faze the animal, he adds.
Although the molecular mechanisms weren’t understood at the time, in 1894 famed neuroscientist Santiago Ramón y Cajal proposed that the brain stores memories and learns facts by strengthening connections between its nerve cells. Cajal thought that these junctions, or synapses, changed shape so that the cells would more firmly grasp one another and, thus, more efficiently conduct signals.
This hypothesis has held up: Today, researchers think that nerve cells strengthen their connections through a process called long-term potentiation. When a synapse is electrically stimulated over and over again, it becomes stable and helps form a memory. This makes sense because “you generally don’t memorize something on the first shot,” Singer says. “Memory isn’t just one event.”

How the brain singles out particular synapses for strengthening, though, is still an unanswered question. Neuroscientists think that mRNAs for various proteins involved in memory formation continuously travel around the branches of nerve cells in packets called granules. These 500-nm-sized globs carry translation factors and other molecular machinery needed to convert the mRNA code into useful proteins. When activated by a nerve impulse, the granules head toward the appropriate synapse, the hypothesis goes. There, they release their cargo to form proteins, such as β-actin, that help reinforce the neuron-neuron junction.
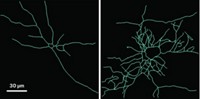
Singer and his colleagues confirmed this model of memory formation with their engineered mice and a two-photon microscope. The researchers stimulated neurons in tissue extracted from their mice and then watched granules containing β-actin mRNA zip along nerve cell branches toward synapses at an average speed of 1.3 µm per second.
Even though neuroscientists have speculated about these events on the basis of experiments with exogenous reporter molecules, the Singer team’s work is a “big step forward,” says Michael Kiebler, head of cell biology at Ludwig Maximilians University, in Munich. “It’s important to be able to visualize mRNA movement, not just to infer it from indirect experiments.”
Singer and his group have also helped “fill a gap in our knowledge about how mRNAs are made available for translation into proteins,” says Erin M. Schuman, managing director of the Max Planck Institute for Brain Research, in Frankfurt.
In one set of experiments, the researchers used a technique called fluorescence in situ hybridization (FISH) to study how mRNAs are packed into the granules. Neuroscientists have assumed that the mRNAs inside the granules are locked up in a way that deters protein translation, Schuman says. “Otherwise, granules would be spinning off copies of protein all the time while en route,” she explains.
Using FISH, Singer and his team added to their nerve cells short, fluorescently labeled strands of RNA that were complementary to the β-actin mRNA already inside. Ten to 15 minutes after the researchers stimulated the nerve cells, twice as many of the added strands hybridized with β-actin mRNA as before cell activation. Within 30 minutes, the boost in hybridization activity disappeared.
These events imply that prior to nerve cell activation, mRNAs ride inside granules in a default, masked configuration, Schuman says. “They also suggest that there’s a window during which mRNA becomes unmasked and can be translated, which is cool.”
Although the studies conducted by Singer’s team have been quite revealing, “it’s going to take more than one or two Science papers to understand how memory works on a molecular scale,” Kiebler says. But the new research has clearly opened exciting avenues for other scientists to explore the complex process of memory formation, he adds.
Singer says he’d like his team to next investigate how much β-actin gets produced by granules after nerve cell stimulation. And the group is already beginning to tackle imaging the brains of live animals: In collaboration with researchers at Howard Hughes Medical Institute’s Janelia Farm Research Campus, the team is putting windows into the skulls of mice to image mRNAs in the animals’ visual cortex.
“I joke to people that we want to show the mouse a picture of a cat and see if the mRNA in its brain gets excited,” Singer says. “It’s a silly analogy, but the point is that we’d like to connect behavior to molecular events.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter