Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Water
Device Mines Precious Phosphorus From Sewage
Water Treatment: A combined osmosis and distillation process could lower the cost of extracting phosphorus from wastewater
by Deirdre Lockwood
January 28, 2014

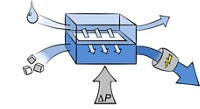
Scientists predict that the scarcity of phosphorus will increase over the next few decades as the growing demand for agricultural fertilizer depletes geologic reserves of the element. Meanwhile, phosphates released from wastewater into natural waterways can cause harmful algal blooms and low-oxygen conditions that can threaten to kill fish. Now a team of researchers has designed a system that could help solve both of these problems. It captures phosphorus from sewage waste and delivers clean water using a combined osmosis-distillation process (Environ. Sci. Technol. Lett. 2014, DOI: 10.1021/ez400189z).
Conventional methods for extracting phosphorus from wastewater use chemicals to precipitate a mineral called struvite from the water. Struvite contains phosphorus, ammonium, and magnesium, and is a useful slow-release fertilizer. Some struvite naturally precipitates out of the water in wastewater treatment plants, causing pipes to clog. But to extract the mineral in amounts that could be sold as fertilizer, engineers must add large amounts of magnesium and increase the pH of the wastewater by adding a strong base. The required amounts of the chemicals are expensive enough that the struvite recovery is seldom cost-effective for utility companies, says Long D. Nghiem, an environmental engineer at the University of Wollongong, in Australia.
To bring down the costs of struvite recovery from wastewater, Nghiem and his colleagues decided to modify a system they recently designed for harvesting freshwater from sewage waste using forward osmosis and membrane distillation (Environ. Sci. Technol. 2013, DOI: 10.1021/es404056e). By tweaking this method, they have reduced the amount of chemicals required to mine struvite from wastewater.
The team’s prototype system has two parts that work in tandem, one for harvesting struvite and the other for recovering freshwater. The first part is a two-sided cell divided by a semipermeable membrane with concentrated wastewater on one side and a highly concentrated salt solution on the other. Osmosis drives water across the membrane from the more dilute wastewater side to the saltier “draw” solution, increasing the concentration of phosphorus and ammonium in the wastewater.
To enhance struvite recovery, the team replaced the usual NaCl draw solution with one of MgCl2. Magnesium ions flow across the membrane to the wastewater side because of the chemical gradient between the two solutions. To preserve charge balance, protons diffuse from the wastewater to the draw solution, increasing the wastewater’s pH. Increasing both the magnesium concentration and the pH of the wastewater creates more favorable conditions for struvite precipitation. To maximize the precipitation, the researchers also added small amounts of MgCl2 and NaOH to the wastewater.
In the second part of the system, where freshwater is recovered, the draw solution is heated and introduced into a cell with a microporous, hydrophobic membrane. Water vapor passes through to the cooler side of the membrane and condenses. A cell with a 1 m2 membrane could distill about 9 L of freshwater per hour. As this distillation process removes freshwater from the MgCl2 solution, it effectively renews the draw solution for continuous operation of the two-part system.
The forward osmosis and distillation process produces 2.4 g of struvite per liter of incoming wastewater. It requires less than one-sixtieth of the MgCl2 and one-fifteenth of the NaOH compared with the conventional method.
“It’s a very innovative and elegant proof of principle,” says Roland D. Cusick, an environmental engineer at the University of Illinois, Urbana-Champaign. But he says the team must overcome some hurdles before the system can be implemented on a commercial scale. In particular, they must address the fouling of the system’s semipermeable membrane, which must be frequently flushed with deionized water to maintain maximum flow rates.
Nghiem’s team is researching ways to limit this fouling and hopes to test the system on a pilot scale within the next few years.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter