Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Coating For Medical Devices Kills Fungi On Contact
Antimicrobials: Functionalized silicone rubber surfaces release drugs when they come in contact with fungi
by Erika Gebel Berg
April 21, 2014

Fungi are everywhere. Fungal films coat surfaces around us, and a fog of microscopic spores permeates the air. These ubiquitous microbes usually cause us no harm, but opportunistic species can take advantage of some people’s compromised immune systems and start dangerous infections. To combat one hotspot for invading fungi, a team of researchers has developed a coating for the rubber surfaces of medical devices that releases antifungal drugs when fungi are present (Biomacromolecules 2014, DOI: 10.1021/bm500257s).
For people with weakened immune systems, such as AIDS patients or those going through chemotherapy, catheters and similar implanted or inserted medical devices can serve as vehicles for pathogenic fungi. Carmen Alvarez-Lorenzo of the University of Santiago de Compostela, in Spain, wanted to develop a way to kill these microbes before they could start a dangerous infection.
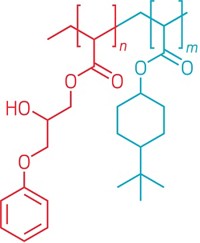
Alvarez-Lorenzo’s strategy involved ergosterol, a cholesterol-like component of fungus cell membranes that isn’t found in mammalian membranes. Several common antifungal medications, such as polyenes, target fungi by binding to ergosterol. The scientist envisioned coating a surface with ergosterol to hold polyene antifungals in place until a pathogenic fungus strolled by. The microbe’s own ergosterol would then bind to and take the drug from the surface, killing itself in the process.

To make the antifungal surfaces, Alvarez-Lorenzo and colleagues used a two-step chemical reaction involving a glycidyl methacrylate linker to attach ergosterol to silicone rubber, a common material in medical devices. Then, they added a polyene antifungal, either natamycin or nystatin, to the surface by dipping the rubber into solutions of the drugs.
The coated rubber showed selective release of the drugs. Less than 10% of the drug was released over two weeks sitting in buffer, demonstrating the surface’s ability to hold onto the antifungals in the absence of fungi. When the researchers added ergosterol-containing liposomes, meant to mimic fungi, the rubber released about 80% of its bound drug over two weeks. To assess whether other membrane types could trigger drug release, the researchers also imitated the surfaces of mammalian cells with cholesterol-containing liposomes. These liposomes caused a significantly slower drug release rate, with 40% of the antifungal freed in two weeks.
The team then tested their coatings against two pathogenic fungi, Candida albicans and Aspergillus fumigatus. They placed the rubber in direct contact with the fungi for 48 hours, and then examined the surface with a microscope for biofilm formation. The surfaces loaded with nystatin, but not natamycin, inhibited growth of both species. In another experiment, the researchers grew C. albicans on the rubber for a day, shook the fungi loose, plated them, and counted the resulting colonies. The number of colonies that grew from the antifungal surface was 1% that of the number from the nonfunctionalized rubber.
Nicholas A. Peppas of the University of Texas, Austin, calls the method novel for its fungi-triggered mechanism of drug release. The work “will have a wide range of applications in the medical and industrial hygiene fields.” For example, the coatings could help control fungal growth on food packaging. Peppas says the researchers still need to prove the materials are safe and effective inside the body. Alvarez-Lorenzo is gearing up for animal studies to test just that.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter