Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Nanovessel Host Leads To Retention Of Configuration In SN2 Reaction
Organic Chemistry: Unexpected reaction may arise from unprecedented supramolecular chemistry
by Bethany Halford
October 15, 2014
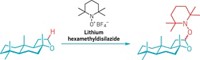
A change to the canon of organic chemistry appears to be in order. Chemists at the University of California, Berkeley, report an SN2 reaction that proceeds with retention of stereochemical configuration (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja508799p).
As students of organic chemistry learn early on, SN2 reactions are characterized by inversion of stereochemical configuration. The incoming nucleophile displaces the leaving group on a carbon atom through a so-called backside attack that flips the arrangement of the carbon’s three other substituents. It’s akin to an umbrella becoming inverted by a strong gust of wind.
Chen Zhao, F. Dean Toste, Kenneth N. Raymond, and Robert G. Bergman discovered they could make the reaction proceed with stereochemical retention quite by accident. They’d come across a clever way of making single diastereomers of these so-called nanovessels and hoped to use them in asymmetric reactions. But when they tried to use the nanovessel for a substitution reaction on a racemic mixture of a benzylic substrate with the hope of getting primarily one enantiomer of product, their results were disappointing.

So, they looked at what happened when they did the same reaction on a single enantiomer of the benzylic substrate. Much to their surprise, the reaction proceeded with up to 90% retention of stereochemical configuration.
“We think that what we’re seeing is a kind of double inversion,” Bergman explains. As the leaving group departs, the π-electrons from one of the naphthalene groups that make up the nanovessel’s walls interact with the backside of the developing carbocation in the benzylic substrate. This sort of interaction has been observed before with neighboring groups within a substrate molecule but never intermolecularly.
“This paper is a great combination of good, old-fashioned sleuthing applied to the frontiers area of supramolecular host-guest catalysis,” comments Ryan A. Shenvi, an organic chemist at Scripps Research Institute, in La Jolla, Calif., who has also tinkered with the canon of SN2 reactions. “The ability to divert the bulk-solvent reactivity of a substrate, the possibility of aromatic ring participation, and the reaction’s sheer coolness all give the project great momentum. It’s easy to imagine many more discoveries snowballing from this single observation,” he says.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter